EXHIBIT 99.1
Published on September 9, 2019
Exhibit 99.1

NASDAQ: EYEG Corporate Presentation

Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s OBG and EGP - 437 product candidates , for the timing and outcome of the Company’s clinical trials, the potential approval to market OBG and EGP - 437 , and the Company’s capital needs . Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation . Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful . The Company may consume more cash than it currently anticipates and faster than projected . Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates . If the U . S . Food and Drug Administration or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them . Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities . If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations . Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K filed with the SEC on March 01 , 2019 . The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law . The Company uses its website ( www . EyeGatePharma . com ), Facebook page ( https : //www . facebook . com/ EyeGatePharma / ), corporate Twitter account ( https : //twitter . com/EyeGatePharma ), and LinkedIn page ( https : //www . linkedin . com/company/ 135892 / ) as channels of distribution of information about the Company and its product candidates . Such information may be deemed material information, and the Company may use these channels to comply with its disclosure obligations under Regulation FD . Therefore, investors should monitor the Company’s website and its social media accounts in addition to following its press releases, SEC filings, public conference calls, and webcasts . The social media channels that the Company intends to use as a means of disclosing the information described above may be updated from time to time as listed on the Company’s investor relations website . Forward Looking Statements 2
3 EyeGate Pharma: Company Highlights Nasdaq: EYEG » Lead product is the Ocular Bandage Gel (OBG), a unique, first - in - class eye drop formulation » Hyaluronic acid, or HA, is a natural substance in the body that promotes wound healing and provides hydration and lubrication . Modified HA is the main ingredient behind OBG. » OBG helps accelerate the healing process of the cornea while hydrating and lubricating the ocular surface, something that no other eye drop can do » Currently in clinical development for two indications: ▪ Wound Healing: final pivotal study initiated; expect top - line data by year - end ▪ Punctate Epitheliopathies (PE): second pilot study initiated; expect top - line data by year - end » Device regulatory pathway; accelerates product development plan and time - to - market
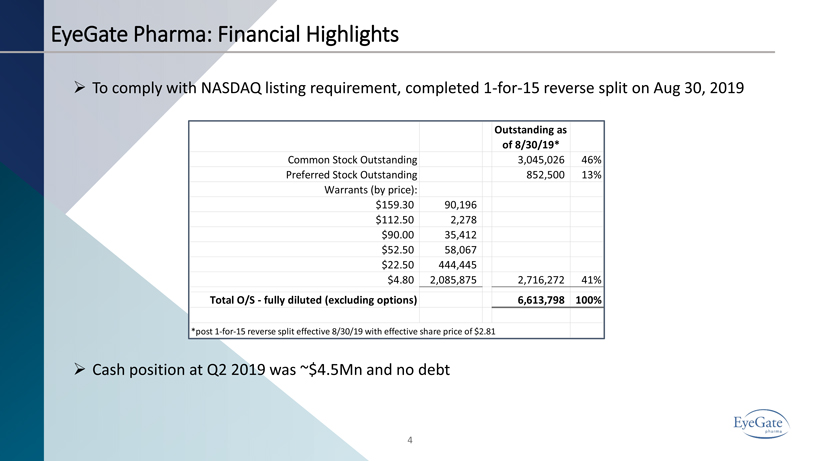
4 EyeGate Pharma: Financial Highlights » To comply with NASDAQ listing requirement, completed 1 - for - 15 reverse split on Aug 30, 2019 » Cash position at Q2 2019 was ~$4.5Mn and no debt Outstanding as of 8/30/19* Common Stock Outstanding 3,045,026 46% Preferred Stock Outstanding 852,500 13% Warrants (by price): $159.30 90,196 $112.50 2,278 $90.00 35,412 $52.50 58,067 $22.50 444,445 $4.80 2,085,875 2,716,272 41% Total O/S - fully diluted (excluding options) 6,613,798 100% *post 1-for-15 reverse split effective 8/30/19 with effective share price of $2.81
Strong & Experienced Leadership Team Stephen From President & Chief Executive Officer Sarah Romano Chief Financial Officer Michael Manzo Vice President of Engineering Lisa Brandano Vice President of Clinical Operations Barbara Wirostko , M.D. Medical Advisor (former Chief Medical Officer) Brenda Mann, PH.D. Vice President of R&D • Has served as President, Chief Executive Officer, and director since October 2005 • Prior roles as Chief Financial Officer at Centelion SAS, an independent biotechnology subsidiary of Sanofi - Aventis, and Director in the Global Healthcare Corporate and Investment Banking Group and Head of European Life Sciences for Bank of America Securities • Joined EyeGate in conjunction with March 2016 acquisition of Jade Therapeutics, where she was a co - founder and served as Chief Scientific Officer • Prior role as Senior Medical Director at Pfizer • Board certified ophthalmologist and a fellow of the American Academy of Ophthalmology • Holds an M.D. from Columbia University; holds a Bachelor’s Degree in Microbiology from Cornell University • Prior roles as Assistant Controller at TechTarget and Corporate Controller at Bowdoin Group, financial reporting positions at SoundBite Communications, Senior Financial Reporting Analyst at Cognex Corporation • Licensed CPA in Massachusetts; holds a Bachelor of Arts in Accounting from College of the Holy Cross and Masters of Accounting from Boston College • Joined EyeGate in October 2006; over 30 years of experience in product development and manufacturing in the medical device industry • Prior roles as President and Chief Operating Officer at Jenline Industries, Ltd., & work with a number of start - up companies in cardiology, radiology, urology and laparoscopic surgery • Co - founder of SentrX Animal Care, focused on veterinary biomaterials and manufacturer of the CMHA - S material • Adjunct associate professor in the Department of Bioengineering at the University of Utah • Founding faculty member of the Keck Graduate Institute of Applied Life Sciences, and now serves on its Advisory Council • Director of the Salt Lake Valley Science and Engineering Fair and a registered patent agent • Prior roles as Assistant Managing Director and Director of Clinical Trial Operations for CATO • Clinical Research experience includes monitoring and project management of various neurology, hematology, and cardiovascular gene therapy studies spanning Phases 1, 2, and 3 • Holds a BS in biology from Emmanuel College; certified clinical research associate (CRA) with the Association of Clinical Research Professionals 5

Ocular Bandage Gel (OBG) Eye Drop
7 Ocular Bandage Gel (OBG) Highlights » Highly purified, crosslinked version of HA in a buffer with no other ingredients » Will be the only prescription HA eye drop in the U.S. » The only covalently crosslinked HA eye drop in the world ▪ This modification of HA prevents degradation providing device regulatory pathway in the U.S. » At 0.75% HA, highest concentration HA eye drop in the world ▪ 5x greater concentration than the HA in Allergan’s Refresh® Repair and J&J’s Blink® (both are non - crosslinked, only available OTC and the HA is an inactive ingredient) » Will be the only eye drop in the U.S. approved/labeled for: ▪ Wound Healing and ▪ Punctate Epitheliopathies (such as dry eye) Over 60 Million Potential Patients in the U.S. Unique first - in - class Hyaluronic Acid (HA) eye drop
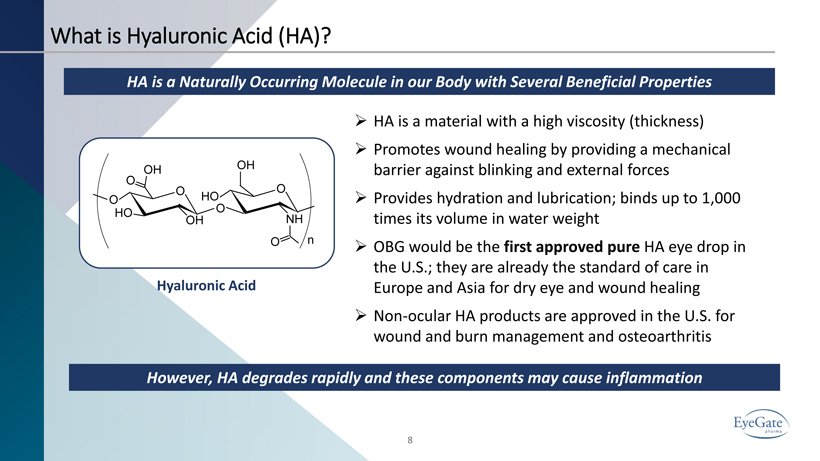
8 What is Hyaluronic Acid (HA)? HA is a Naturally Occurring Molecule in our Body with Several Beneficial Properties Hyaluronic Acid » HA is a material with a high viscosity (thickness) » Promotes wound healing by providing a mechanical barrier against blinking and external forces » Provides hydration and lubrication; binds up to 1,000 times its volume in water weight » OBG would be the first approved pure HA eye drop in the U.S.; they are already the standard of care in Europe and Asia for dry eye and wound healing » Non - ocular HA products are approved in the U.S. for wound and burn management and osteoarthritis However, HA degrades rapidly and these components may cause inflammation O O HO HO OH OH OH O O O O NH n
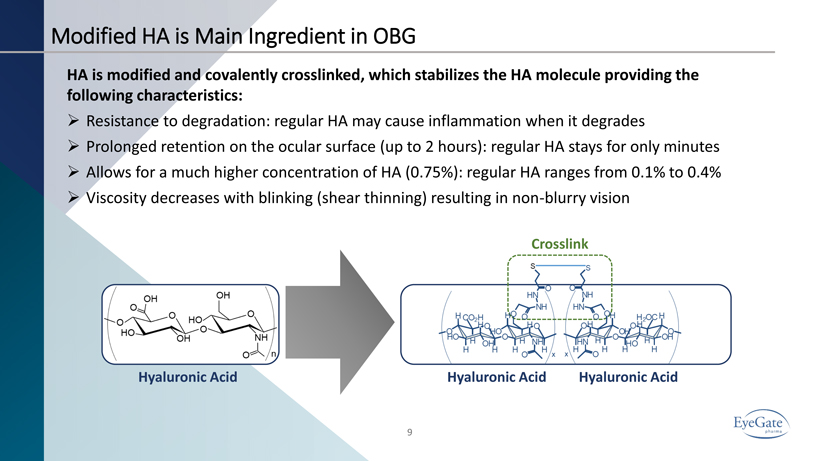
Hyaluronic Acid Hyaluronic Acid Hyaluronic Acid 9 Crosslink Modified HA is Main Ingredient in OBG HA is modified and covalently crosslinked, which stabilizes the HA molecule providing the following characteristics: » Resistance to degradation: regular HA may cause inflammation when it degrades » Prolonged retention on the ocular surface (up to 2 hours): regular HA stays for only minutes » Allows for a much higher concentration of HA (0.75%): regular HA ranges from 0.1% to 0.4% » Viscosity decreases with blinking (shear thinning) resulting in non - blurry vision

10 OBG: Efficacy Demonstrated in Various Studies A B Molly: 12 year old cat with a non - healing corneal defect A. Non - healing at 42 days B. Ulcer healing after 12 days of using 0.75% CMHA - S Animal Studies » Post traumatic corneal stromal ulcers (real world dogs and cats) » Dry eye (veterinary dogs who failed topical cyclosporine) » Corneal abrasion and alkali burn injuries (rabbit models) Additionally, positive results from 3 human clinical studies: 2 for Wound Healing and 1 for PE » Demonstrated ability of an eye drop to close large epithelial wounds as well as a bandage contact lens » Showed statistical significant reduction in symptoms vs. control for patients with history of dry eye (PE study) » Demonstrating effectiveness in two extremes of corneal epitheliopathies: large defects to small micro defects
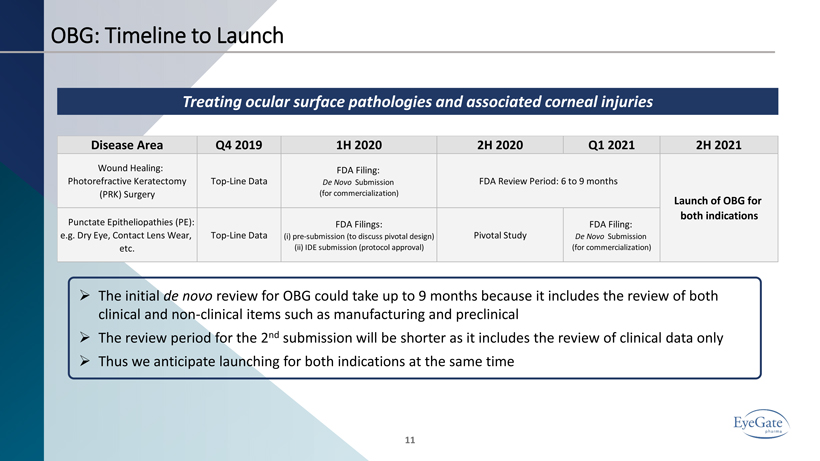
OBG: Timeline to Launch 11 Treating ocular surface pathologies and associated corneal injuries » The initial de novo review for OBG could take up to 9 months because it includes the review of both clinical and non - clinical items such as manufacturing and preclinical » The review period for the 2 nd submission will be shorter as it includes the review of clinical data only » Thus we anticipate launching for both indications at the same time Disease Area Q4 2019 1H 2020 2H 2020 Q1 2021 2H 2021 Wound Healing: Photorefractive Keratectomy (PRK) Surgery Top-Line Data FDA Filing: De Novo Submission (for commercialization) Punctate Epitheliopathies (PE): e.g. Dry Eye, Contact Lens Wear, etc. Top-Line Data FDA Filings: (i) pre-submission (to discuss pivotal design) (ii) IDE submission (protocol approval) Pivotal Study FDA Filing: De Novo Submission (for commercialization) FDA Review Period: 6 to 9 months Launch of OBG for both indications
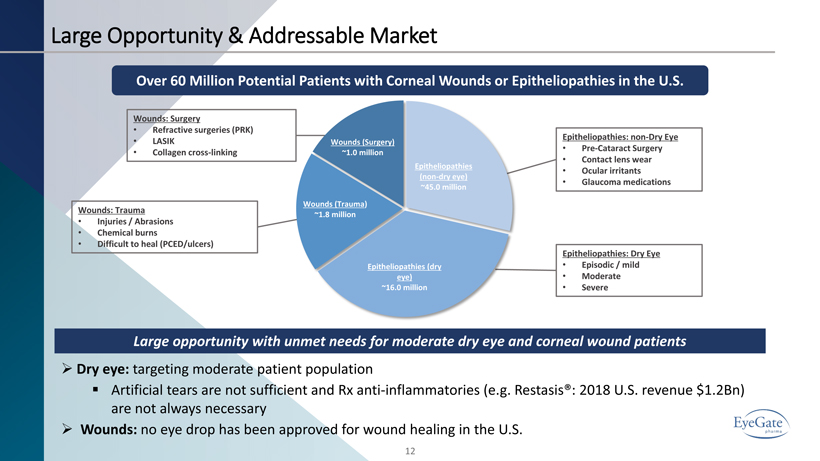
Large Opportunity & Addressable Market 12 Wounds: Surgery • Refractive surgeries (PRK) • LASIK • Collagen cross - linking Epitheliopathies: Dry Eye • Episodic / mild • Moderate • Severe Epitheliopathies: non - Dry Eye • Pre - Cataract Surgery • Contact lens wear • Ocular irritants • Glaucoma medications Over 60 Million Potential Patients with Corneal Wounds or Epitheliopathies in the U.S. Epitheliopathies (non - dry eye) ~45.0 million Epitheliopathies (dry eye) ~16.0 million Wounds (Trauma ) ~1.8 million Wounds (Surgery) ~1.0 million Wounds: Trauma • Injuries / Abrasions • Chemical burns • Difficult to heal (PCED/ulcers) Large opportunity with unmet needs for moderate dry eye and corneal wound patients » Dry eye: targeting moderate patient population ▪ Artificial tears are not sufficient and Rx anti - inflammatories (e.g. Restasis®: 2018 U.S. revenue $1.2Bn) are not always necessary » Wounds: no eye drop has been approved for wound healing in the U.S.

13 Clinical and Commercial Strategy » OBG heals the epithelial cells covering the cornea; there are many different types and sizes of epithelial defects from surgically induced to non - healing ulcers to micro - abrasions » Primary Objective: approval for treating all epithelial defects without doing a pivotal study for each indication ▪ FDA has agreed that OBG should work in all types and sizes of epithelial defects ▪ FDA has agreed to allow approval of multiple indications under the umbrella of punctate epitheliopathies (PE) ▪ PE is not a single indication; it covers multiple indications with a similarity of micro - abrasions to the cornea such as dry eye, contact lens wear (tight lens syndrome), etc. » Secondary Objective: approval for commercialization as quickly as possible ▪ PE is largest financial opportunity, but regulatory pathway not clear ▪ Determining PE endpoint is complicated (e.g. sign, symptom, timing, dosing, etc.) ▪ Although smaller financial opportunity, wound healing via PRK surgery has clear and quickest path to approval ▪ Once first de novo is approved, second approval for a device is quick » With these two approvals we are covering the majority of patients that have corneal epitheliopathies ▪ Final areas to cover are traumatic wounds and PCEDs like non - healing ulcers

Wound Healing Studies
15 Demonstrating Wound Healing in PRK Patients » PRK is a surgical correction of refractive errors for patients who are not suitable candidates for LASIK due to: ▪ Inadequate corneal thickness ▪ Larger pupil size ▪ History of keratoconjunctivitis sicca (KCS) ▪ Anterior basement membrane disease » PRK involves controlled mechanical removal of corneal epithelium with subsequent lasering of stroma » Although PRK yields excellent visual results, common complications include: ▪ Post - operative pain ▪ Risk of corneal infection prior to re - epithelialization ▪ Corneal haze formation ▪ Decreased contrast sensitivity ▪ Slower visual recovery » Enabling the epithelium to heal faster may mitigate the immediate peri - operative complications as well as improve the longer visual term outcomes About Photorefractive Keratectomy (PRK)

EyeGate’s Wound Healing Clinical Studies Objective is to demonstrate acceleration of the healing process 15 Design for both of the pilot studies: • Treatment Group 1: OBG 4x/day (QID) for 14 days • Treatment Group 2: Study 1 (OBG combined with BCL), Study 2 (OBG 8x/day for 3 days followed by QID for 11 days) • Control Group: Standard of care (bandage contact lens + artificial tears) • Study 2 was masked using a reading center (Tufts): digital photography of fluorescein stained slit lamp photos » PRK population ideal for clinical development: ▪ Large population (~700,000 LASIK/PRK surgeries per year in the U.S.) ▪ Large wound (9mm), same size for all patients and know time zero ▪ Healthy eyes required and time to healing well established » Standard - of - care is a Bandage Contact Lens (BCL); can result in subsequent erosion of epithelium » Two clinical studies completed demonstrating acceleration of healing vs standard - of - care ▪ Primary outcome = percentage of patients healed at Day 3
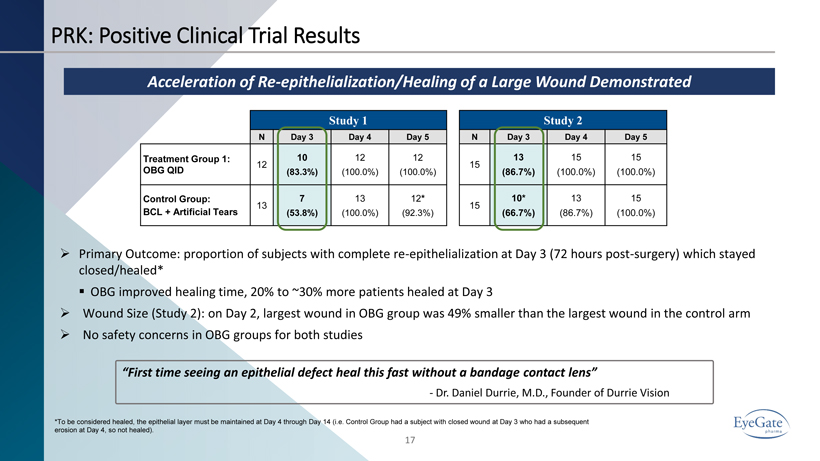
PRK: Positive Clinical Trial Results 17 Acceleration of Re - epithelialization/Healing of a Large Wound Demonstrated Study 1 N Day 3 Day 4 Day 5 Treatment Group 1: OBG QID 12 10 (83.3%) 12 (100.0%) 12 (100.0%) Control Group: BCL + Artificial Tears 13 7 (53.8%) 13 (100.0%) 12 * (92.3%) Study 2 N Day 3 Day 4 Day 5 15 13 (86.7%) 15 (100.0%) 15 (100.0%) 15 10* (66.7%) 13 (86.7%) 15 (100.0%) » Primary Outcome: proportion of subjects with complete re - epithelialization at Day 3 (72 hours post - surgery) which stayed closed/healed* ▪ OBG improved healing time, 20% to ~30% more patients healed at Day 3 » Wound Size (Study 2): o n Day 2, largest wound in OBG group was 49% smaller than the largest wound in the control arm » No safety concerns in OBG groups for both studies *To be considered healed, the epithelial layer must be maintained at Day 4 through Day 14 (i.e. Control Group had a subject w ith closed wound at Day 3 who had a subsequent erosion at Day 4, so not healed). “First time seeing an epithelial defect heal this fast without a bandage contact lens” - Dr. Daniel Durrie, M.D., Founder of Durrie Vision
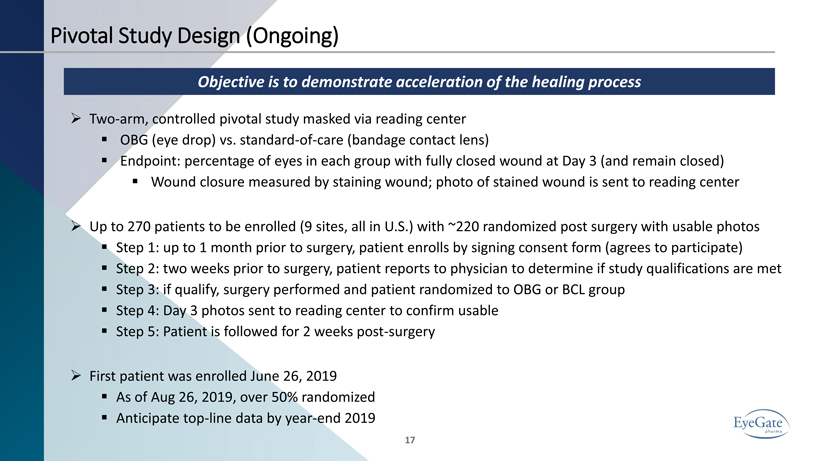
Pivotal Study Design (Ongoing) Objective is to demonstrate acceleration of the healing process 17 » Two - arm, controlled pivotal study masked via reading center ▪ OBG (eye drop) vs. standard - of - care (bandage contact lens) ▪ Endpoint: percentage of eyes in each group with fully closed wound at Day 3 (and remain closed) ▪ Wound closure measured by staining wound; photo of stained wound is sent to reading center » Up to 270 patients to be enrolled (9 sites, all in U.S.) with ~220 randomized post surgery with usable photos ▪ Step 1: up to 1 month prior to surgery, patient enrolls by signing consent form (agrees to participate) ▪ Step 2: two weeks prior to surgery, patient reports to physician to determine if study qualifications are met ▪ Step 3: if qualify, surgery performed and patient randomized to OBG or BCL group ▪ Step 4: Day 3 photos sent to reading center to confirm usable ▪ Step 5: Patient is followed for 2 weeks post - surgery » First patient was enrolled June 26, 2019 ▪ As of Aug 26, 2019, over 50% randomized ▪ Anticipate top - line data by year - end 2019

PE Study
20 About Punctate Epitheliopathies (PE) » Punctates are a sign of epithelial compromise and are characterized by a breakdown or damage of the epithelium of the cornea and therefore stain positively with fluorescein » Patients may present with non - specific symptoms such as tearing, foreign body sensation, photophobia, and blurred vision » PE is associated with many pathologic ocular inflammatory conditions, which can include: ▪ dry eye ▪ acute and chronic bacterial and viral conjunctivitis ▪ Trauma/corneal abrasions ▪ contact lens wear (tight lens syndrome) ▪ chemical irritation and burns ▪ diabetic and infectious neuropathies ▪ chemotherapy ▪ Corneal medications ▪ eyelid malposition with secondary exposure keratopathy
EyeGate’s Punctate Epitheliopathies (PE) Study Objective is to reduce corneal staining and improve the accompanying symptoms 20 Pilot Study Design ▪ 30 subjects: 6 week study (2 week wash - out followed by 4 weeks of treatment) • 2 week wash - out period: all 30 patients stop taking prescription eye drops and receive rewetting eye drops* only • 4 week treatment period: OBG QID vs rewetting eye drops* (15 patients per group) ▪ Patients must have staining score ≥4 at beginning of wash - out (Day - 14) period and beginning of treatment (Day 0) period *Bausch & Lomb Sensitive Eyes rewetting drops: A sterile buffered, isotonic, aqueous solution that contains boric acid, sodiu m b orate, sodium chloride and poloxamine; preserved with sorbic acid 0.15% and edetate disodium 0.1% » Nothing currently approved for the treatment of PE in the U.S. ▪ Large population (millions of patients in the U.S.), easy to recruit ▪ Many causes, a large one being dry eye, which is not a homogenous population » One clinical study completed demonstrating reduction in staining of central cornea and a statistically significant improvement in symptoms » Primary outcome was based on fluorescein staining of the cornea; not an objective measurement » Combination of non - homogenous population and subjective measurement resulted in mixed outcome with small study » FDA has agreed to discuss alternative endpoints (i.e. objective ones)
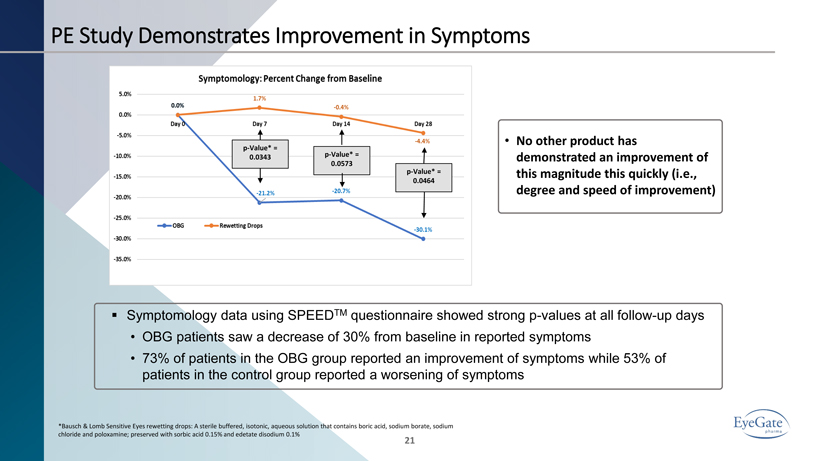
21 ▪ Symptomology data using SPEED TM questionnaire showed strong p - values at all follow - up days • OBG patients saw a decrease of 30% from baseline in reported symptoms • 73% of patients in the OBG group reported an improvement of symptoms while 53% of patients in the control group reported a worsening of symptoms *Bausch & Lomb Sensitive Eyes rewetting drops: A sterile buffered, isotonic, aqueous solution that contains boric acid, sodiu m b orate, sodium chloride and poloxamine; preserved with sorbic acid 0.15% and edetate disodium 0.1% p - Value* = 0.0343 p - Value* = 0.0573 p - Value* = 0.0464 PE Study Demonstrates Improvement in Symptoms • No other product has demonstrated an improvement of this magnitude this quickly (i.e., degree and speed of improvement)
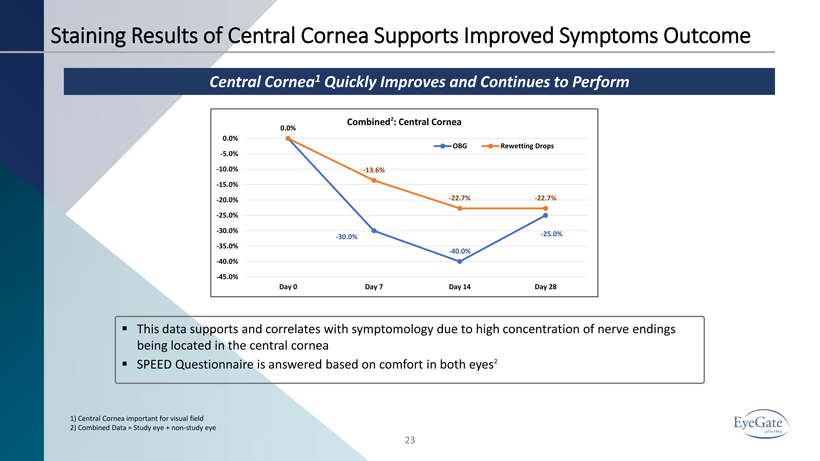
Staining Results of Central Cornea Supports Improved Symptoms Outcome 23 Central Cornea 1 Quickly Improves and Continues to Perform 1) Central Cornea important for visual field 2) Combined Data = Study eye + non - study eye ▪ This data supports and correlates with symptomology due to high concentration of nerve endings being located in the central cornea ▪ SPEED Questionnaire is answered based on comfort in both eyes 2 0.0% - 30.0% - 40.0% - 25.0% 0.0% - 13.6% - 22.7% - 22.7% -45.0% -40.0% -35.0% -30.0% -25.0% -20.0% -15.0% -10.0% -5.0% 0.0% Day 0 Day 7 Day 14 Day 28 Combined 2 : Central Cornea OBG Rewetting Drops
Objective is to evaluate several performance endpoints for use in pivotal study 23 » Two - arm, controlled study ▪ OBG vs. artificial tears ▪ Patient is their own control: both eyes must qualify where one eye is randomized to OBG and the other eye to artificial tears ▪ Randomizing up to 20 patients (40 eyes, 20 per group) at 2 sites, both in the U.S. ▪ Initiation recently completed; beginning search for patients to enroll ▪ Anticipate top - line data by year - end 2019 2 nd Pilot Study Design (Ongoing)

Thank You NASDAQ: EYEG Contact Us Joseph Green Edison Advisors Investor Relations Tel: (646) 653 - 7030 E - mail: jgreen@edisongroup.com