10-K: Annual report pursuant to Section 13 and 15(d)
Published on March 2, 2018
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | Annual report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended December 31, 2017
or
| ¨ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to
Commission File Number 001-36672
EYEGATE PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 98-0443284 |
|
(State or other jurisdiction of Incorporation or organization) |
(I.R.S. Employer Identification No.) |
271 Waverley Oaks Road
Suite 108
Waltham, MA 02452
(Address of Principal Executive Offices, including zip code)
(781) 788-9043
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Common Stock, $0.01 par value
Warrants to Purchase Common Stock
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ¨ NO x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ¨ NO x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES x NO ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES x NO ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large Accelerated filer | ¨ | Accelerated filer | ¨ |
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x |
| Emerging growth company | x |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ¨ NO x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of June 30, 2017 was approximately $19,108,212. Shares of the registrant’s common stock held by each officer and director and each person known to the registrant to own 10% or more of the outstanding voting power of the registrant have been excluded in that such persons may be deemed affiliates. This determination of affiliate status is not a determination for other purposes.
At February 28, 2018, there were 17,257,255 shares of the registrant’s common stock issued and outstanding.
EYEGATE PHARMACEUTICALS, INC.
Table of Contents
ANNUAL REPORT ON FORM 10-K
For the Year Ended December 31, 2017
INDEX
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains statements that are not statements of historical fact and are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), each as amended. The forward-looking statements are principally, but not exclusively, contained in “Item 1: Business” and “Item 7: Management’s Discussion and Analysis of Financial Condition and Results of Operations.” These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements about management’s confidence or expectations, and our plans, objectives, expectations and intentions that are not historical facts. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “goals,” “sees,” “estimates,” “projects,” “predicts,” “intends,” “think,” “potential,” “objectives,” “optimistic,” “strategy,” and similar expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss many of these risks in detail under the heading “Item 1A. Risk Factors” beginning on page 25 of this Annual Report on Form 10-K. You should carefully review all of these factors, as well as other risks described in our public filings, and you should be aware that there may be other factors, including factors of which we are not currently aware, that could cause these differences. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this report. We may not update these forward-looking statements, even though our situation may change in the future, unless we have obligations under the federal securities laws to update and disclose material developments related to previously disclosed information.
EyeGate Pharmaceuticals, Inc. is referred to herein as “we,” “our,” “us,” and “the Company.”
| 1 |
| Item 1. | Business. |
Overview
We are a clinical-stage specialty pharmaceutical company that is focused on developing and commercializing products for treating diseases and disorders of the eye. We accomplish this by leveraging our two proprietary platform technologies, crosslinked thiolated carboxymethyl hyaluronic acid (“CMHA-S”) and our iontophoresis drug delivery system.
We are developing products using CMHA-S, a modified form of the natural polymer hyaluronic acid, which is a gel that possesses unique physical and chemical properties such as hydrating and healing properties when applied to the ocular surface. The ability of CMHA-S to adhere longer to the ocular surface, resist degradation and protect the ocular surface makes it well-suited for treating various ocular surface injuries. Our first CMHA-S-based product, the EyeGate Ocular Bandage Gel (“OBG”), has completed a pilot trial where we announced positive top-line data. OBG is a topically-applied eye drop formulation that is being developed under the 510(k) De Novo path for devices submitted for marketing clearance to the U.S. FDA.
The CMHA-S platform is based on hyaluronic acid (“HA”), a naturally occurring polymer that is important in many physiological processes, including wound healing, tissue homeostasis, and joint lubrication. To create hydrogels, the HA is modified to create CMHA-S that is then cross-linked together through the thiol groups. Some products employ disulfide cross-linking while others utilize a Polyethylene Glycol Diacrylate, or PEGDA, cross-linker. Cross-linking slows degradation of the HA backbone and provides a matrix for incorporating therapeutic agents. Variations in the number of thiols per molecule, the molecular weight of the polymer, the concentration of the polymer, the type of cross-linking, and incorporation of active ingredients, provides a highly versatile platform that can be tailored to a specific application. CMHA-S can be formulated as gels or films.
Our first CMHA-S-based product candidate, the EyeGate Ocular Bandage Gel (“OBG”), is a topically-applied eye drop formulation that has completed its first-in-man clinical trial. We announced positive top-line data from the initial pilot trial evaluating the ability of EyeGate OBG to accelerate ocular surface re-epithelialization following photorefractive keratectomy (“PRK”). The EyeGate OBG eye drop creates a thin, durable and protective coating to the damaged surface of the eye, serving to facilitate and accelerate corneal re-epithelization. The EyeGate OBG is intended for the management of corneal epithelial wounds, defects, and epitheliopathies.
Preclinical studies suggest that the specific CMHA-S chemical modification comprising the EyeGate OBG creates a favorable set of attributes, including prolonged retention time on the ocular surface, and a smooth continuous clear barrier without blur that can minimize mechanical lid friction, reduce repeat injury, and mechanically protect the ocular surface, allowing accelerated corneal re-epithelization.
The gel is presently available commercially as a veterinary device indicated for use in the management of superficial corneal ulcers. Manufactured by SentrX Animal Care and sold in the U.S. by Bayer Animal Health as Remend® Corneal Repair, the product has been used successfully for five years in dogs, cats and horses, without adverse effects. The composition of the veterinary product is identical to that of the EyeGate OBG. We do not have the rights to the CMHA-S platform for animal health or veterinary medicine.
In addition, we are developing EGP-437, which incorporates a reformulated topically active corticosteroid, Dexamethasone Phosphate, that is delivered into the ocular tissues through our proprietary innovative drug delivery system, the EyeGate® II Delivery System. EGP-437 is being developed under the 505(b)(2) New Drug Application, or NDA, regulatory pathway for drugs submitted for approval to the U.S. Food and Drug Administration, or FDA, which enables an applicant to rely, in part, on the FDA’s findings of safety and efficacy for an existing product, or published literature, in support of its NDA.
The EyeGate® II Delivery System features a compact, elegant, and easy-to-use device that we believe has the potential to deliver drugs non-invasively and quickly into the ocular tissues through the use of iontophoresis, which can accelerate the onset of action, dramatically reduce treatment frequency versus eye drops and sustain therapeutic effect. Iontophoresis employs the use of a low electrical current that promotes the migration of a charged drug substance across biological membranes. The current produces ions, which through electrorepulsion, drive a like-charged drug substance into the tissues. The EyeGate® II Delivery System is easy-to-use, only takes a few minutes to employ and more than 3,000 treatments have been administered in clinical trials.
We are developing EGP-437 for the treatment of various inflammatory conditions of the eye, including the treatment of ocular inflammation and pain in post-surgical cataract patients and anterior uveitis, a debilitating form of intraocular inflammation of the anterior portion of the uvea, such as the iris and/or ciliary body. Based on guidance provided by the FDA, we expect that if the planned confirmatory Phase 3 trial of EGP-437 in anterior uveitis meets non-inferiority criteria, data from this trial along with data from our previously completed Phase 3 trial in anterior uveitis will be sufficient to support an NDA filing. We also believe, based on guidance provided by the FDA, that the design of the planned confirmatory Phase 3 anterior uveitis trial is acceptable and that the nonclinical work completed to date is sufficient to support an NDA filing.
| 2 |
We have entered into two exclusive global license agreements with a subsidiary of Valeant Pharmaceuticals International, Inc. (“Valeant”) through which we granted Valeant exclusive, worldwide commercial and manufacturing rights to our EyeGate® II Delivery System and EGP-437 combination product, or the Product, in the fields of anterior uveitis and ocular iontophoretic treatment for post-operative ocular inflammation and pain in ocular surgery patients, as well as a right of last negotiation to license the Product for other indications. We are responsible for the development of the Product in the U.S. for the indication of anterior uveitis, together with the costs associated therewith. Valeant has the right to develop the Product in the fields outside of the U.S. and has agreed to fund 100% of any costs associated therewith.
Our Strategy
Our goal is to become a leading specialty pharmaceutical company focused on developing and commercializing products for treating diseases and disorders of the eye. The key elements of this strategy are to:
| · |
Continue clinical development of our EyeGate OBG device for the treatment of corneal epithelial defects. We completed our first-in-man trial enrolling subjects with a 9mm corneal wound, a large corneal epithelial defect, post photorefractive keratectomy (PRK) surgery and released positive top-line data in the first quarter of 2017. We expect to initiate a masked controlled pilot trial in the first half of 2018. |
|
| · |
Initiate clinical development of our EyeGate OBG device for the treatment of punctate epitheliopathies. We anticipate submitting a second IDE in the first quarter of 2018 to begin a clinical trial focused on treating patients with punctate epitheliopathies as confirmed by fluorescein staining of the cornea. We expect to initiate a masked controlled pilot trial in the first half of 2018. |
|
| · |
Continue to analyze the data from our recently completed Phase 2b trial with our EGP-437 Combination Product for the treatment of inflammation and pain post cataract surgery. We have recently completed enrollment of a 100 subject double-masked placebo controlled Phase 2b trial and announced topline data for this trial in the first quarter of 2018. Although EGP-437 demonstrated a higher rate of success compared to vehicle at all time points, the co-primary endpoints of proportion of subjects with an anterior chamber cell (ACC) count of zero at day 7 and the proportion of subjects with a pain score of zero at day 1 did not show statistical significance. The efficacy results for the absence of inflammatory cells in the EGP-437 treatment group met our expectations, but the vehicle group response was better than anticipated. We will continue to review the data to determine next steps and to continue evaluating EGP-437 for the reduction of pain and inflammation following ocular surgery. |
|
| · |
Continue clinical development of our EGP-437 Combination Product for the treatment of noninfectious anterior uveitis. We have initiated and continue enrolling patients for the confirmatory Phase 3 trial evaluating the safety and efficacy of our EGP-437 Combination Product for the treatment of noninfectious anterior uveitis. Based on our estimates regarding subject enrollment, we expect to have topline data for this trial in the third quarter of 2018. |
|
| · | Utilize the EyeGate iontophoresis expertise to expand our drug delivery platform for the treatment of eye diseases. Our initial platform, the EyeGate® II Drug Delivery System, is an in-office treatment performed by an eye care giver. We plan to develop a system based on iontophoresis that could be applied at home by the patient. This would be ideal for the treatment of certain chronic ocular diseases where less frequent visits to the eye care givers office are required. |
| · | Pursue other strategic collaborations. We plan to evaluate opportunities to enter into collaborations that may contribute to our ability to advance our drug delivery platform and product candidates and to progress concurrently a range of discovery and development programs. We also plan to evaluate opportunities to in-license or acquire the rights to other products, product candidates or technologies for the treatment of eye diseases. |
| 3 |
Ophthalmic Market Opportunity
Ophthalmology is a specialty market with commercial and regulatory dynamics that make it possible for small or medium sized companies like us to develop and commercialize products on our own. We believe that the specialists in the U.S. who treat ocular diseases are sufficiently concentrated that we could effectively promote our products with a specialty sales and marketing group.
EyeGate OBG
The EyeGate OBG is a synthetic biocompatible cross-linked thiolated carboxymethyl hyaluronic acid (CMHA-S) hydrogel capable of coating the ocular surface and designed to resist degradation under conditions present in the eye. This prolongs residence time of the bandage on the ocular surface, thereby addressing the limitations of current non-cross-linked hyaluronic acid formulations. Additionally, cross-linking allows the product’s viscosity to be modified to meet optimum ocular needs. The increased viscosity and non-covalent muco-adhesive interfacial forces improve residence time in the tear film, thus providing a coating that aids and promotes re-epithelization of the ocular surface via physical protection.
The EyeGate OBG exhibits significant shear thinning properties. This feature allows the CMHA-S polymer to act as a more concentrated, viscous barrier at low shear rates in a resting tear film, but also as a lower resistance fluid (therefore thinned) during high shear events such as blinking. This property enables better residence time and a more favorable ocular surface coating with less optical blur. This should enhance ocular surface protection and patient comfort.
The EyeGate OBG has been shown to provide a mechanical barrier that aids in the management of corneal epithelial defects and accelerates re-epithelization in both preclinical studies and in clinical ophthalmic veterinary use. As such, photorefractive keratectomy (PRK) surgery was chosen as the subject population which is best suited to demonstrate this effect. PRK is an efficacious alternative to patients seeking surgical correction of refractive errors who are not suitable candidates for laser in situ keratomileusis (LASIK) due to inadequate corneal thickness, larger pupil size, history of keratoconjunctivitis sicca (KCS), or anterior basement membrane disease. The primary effectiveness endpoint for this initial pilot trial was time to re-epithelization of a large epithelial defect following PRK surgery. We completed the initial trial and announced positive top-line data in the first quarter of 2017. We anticipate initiating our next pilot trial in the first half of 2018.
We believe that the EyeGate OBG can be used for the management of a variety of large and small corneal epithelial defects including Punctate Epitheliopathies. Punctate Epitheliopathies are an early sign of epithelial compromise and are associated with a variety of pathologic ocular inflammatory conditions including ocular causes, as well as systemic diseases. This ocular surface condition is common and may represent areas of epithelial cell damage and loss and therefore stain positively with fluorescein. Causes can include dry eye, acute and chronic bacterial and viral conjunctivitis, trauma, contact lens wear (tight lens syndrome), chemical irritation and burns, diabetic and infectious neuropathies, chemotherapy and corneal abrasion. We plan on submitting a second IDE to the FDA in the first quarter of 2018 for the development of EyeGate OBG for treating Punctate Epitheliopathies. We anticipate initiating our first pilot trial in the first half of 2018.
EyeGate® II Delivery System and EGP-437
Delivery of therapeutic agents using ocular iontophoresis has been of interest as a means of non-invasively achieving higher drug levels within the eye by promoting the migration of a charged drug substance across biological membranes with a low electrical current. The EyeGate® II Delivery System applicator utilizes an inert electrode, which stimulates the electrolysis of water to produce ions (hydroxide or hydronium), which via electrorepulsion, drive a like-charged drug substance into the ocular tissues. The EyeGate® II Delivery System delivery platform requires custom pharmaceutical formulations to enable delivery efficiency and safety while allowing for potential novel intellectual property. The data from multiple clinical trials suggests that EGP-437 does not significantly raise mean intraocular pressure, or IOP, at the time points evaluated during the study period.
Many front of the eye diseases such as cataract surgery and non-infectious anterior uveitis are acute inflammatory conditions. The current standard of care to treat ocular surface and anterior segment inflammation is patient administered corticosteroids in the form of eye drops. Topical corticosteroids suffer from a number of drawbacks including low ocular bioavailability, rapid clearance and steroid-related side effects including elevated IOP. We believe that our EGP-437 Combination Product has the potential to address these unmet needs by providing in-office treatments given by the eye care provider thereby mitigating the patient compliance issues and substantially reducing the burden of care.
Currently, the only primary route of administration for drugs treating retinal diseases is through intravitreal injection into the vitreous of the eye. These injections must be given as frequently as once per month when treating chronic diseases like macular degeneration. Unfortunately, there are known drawbacks associated with administering intravitreal injections, including safety risks, adverse patient experience and being time- and labor-intensive to administer. Data from our Phase 1b/2a proof-of-concept macular edema trial suggests that iontophoresis can non-invasively deliver EGP-437 to the back of the eye. The non-invasive delivery of EGP-437 has demonstrated a positive response in some patients with macular edema.
| 4 |
Current Targeted Indications
EyeGate OBG: Large Corneal Epithelial Defects
The EyeGate OBG provides a thin coating to the surface of the eye, serving as a protectant to facilitate and accelerate corneal re-epithelization. EyeGate conducted a randomized masked, prospective study of the safety and performance of the EyeGate Ocular Bandage Gel, a 0.75% crosslinked Hyaluronic Acid applied topically for accelerating re-epithelization of large corneal epithelial defects resulting from photorefractive keratectomy (PRK) used in combination with and without a bandage contact lens.
Photorefractive keratectomy (PRK) is an efficacious alternative to patients seeking surgical correction of refractive errors who are not suitable candidates for laser in situ keratomileusis (LASIK) due to inadequate corneal thickness, larger pupil size, history of keratoconjunctivitis sicca (KCS), or anterior basement membrane disease. PRK involves controlled mechanical removal of corneal epithelium with subsequent excimer laser photoablation of the underlying Bowman’s layer and anterior stroma, including the subepithelial nerve plexus.
The military prefers PRK as a refractive surgery due to the stability of the PRK incision and the absence of risk for flap dislocation during military active duty. Although this procedure yields desirable visual acuity results, common complications of the procedure include post-operative pain secondary to the epithelial defects, risk of corneal infection prior to re-epithelization of the large epithelial defect, corneal haze formation, decreased contrast sensitivity, and slower visual recovery.
EyeGate OBG: Punctate Epitheliopathies
Punctate Epitheliopathies, or PE, are an early sign of epithelial compromise and are associated with a variety of pathologic ocular inflammatory conditions including ocular causes, as well as systemic diseases. This ocular surface condition is common and may represent areas of epithelial cell damage and loss and therefore stain positively with fluorescein. PE is characterized by a breakdown or damage of the epithelium of the cornea in a pinpoint pattern, which can be seen with examination with a slit-lamp. Patients may present with non-specific symptoms such as red eye, tearing, foreign body sensation, photophobia and burning. Causes can include dry eye, acute and chronic bacterial and viral conjunctivitis, trauma, contact lens wear (tight lens syndrome), chemical irritation and burns, diabetic and infectious neuropathies, chemotherapy and corneal abrasion.
Standard of care treatments are aimed at attempting to heal these punctate micro defects and/or epitheliopathies and can include increasing humidity, artificial tears, lubricants and ointments and in severe cases can even utilize bandage contact lens, antibiotics and amniotic membrane graphs, as well as treating the underlying cause with topical anti-inflammatory and T cell modulators. The endpoint of treatment is to re-epithelize the cornea and resolve the corneal staining. Resolution of the corneal staining are frequently measured by scales such as the National Eye Institute Scale (NEI) or Oxford scale. These standardized and validated scales have been developed to help score and measure these defects. Often these current treatments fall short as they are ineffective in protecting and enabling corneal re-epithelization. The artificial tears have limited residence time and often do nothing to mechanically protect the cornea and create an environment that can accelerate corneal reepithelization and resolve staining. Furthermore, many of the ointments and gels, although offering better residence time, are thicker and blur vision, thus making them less attractive for day time use.
The EyeGate OBG, once applied to the eye, forms a thin layer that protects the eye to promote re-epithelization in the management of a variety of large and small corneal epithelial defects including PE.
EGP-437: Cataract Surgery
Cataracts are the leading cause of blindness worldwide, and there are more than 24 million people age 40 and older who have cataracts in the U.S. alone, according to the Vision Problems in the U.S. report from Prevent Blindness. A cataract is a clouding of the lens in the eye that affects vision. Most cataracts are related to aging and are very common in older people. By age 80, more than half of the U.S. population either have a cataract or have had cataract surgery. Cataract surgery is the most common surgical procedure in the population aged over 65 years. There are approximately three million cataract surgeries performed per year in the U.S. As the technology of cataract surgery has progressed, so too, has the increased patient demand for excellent vision and safety after the procedure, but visual rehabilitation after cataract surgery is sometimes delayed by the inflammatory processes that are induced by phacoemulsification where the eye’s internal lens is emulsified with an ultrasonic hand piece and aspirated from the eye. Inflammation is induced in all cataract surgery by the mechanical transmission of energy into the eye, disruption of cell membranes, and the normal healing process. Postoperative topical corticosteroids are used routinely to reduce inflammation and improve visual outcomes after cataract surgery. Despite their use, transient corneal edema is one of the major factors hindering the improvement of vision in the first days after surgery, and cystoid macula edema may reduce quality of vision for weeks and months after the procedure. Therefore, reducing inflammation and its potential damage to the corneal endothelium and retina is a high priority for the ophthalmic surgeon.
| 5 |
EGP-437: Non-Infectious Anterior Uveitis
Uveitis is a general term for inflammation of the uveal tract and encompasses a wide range of etiologies. It may be iodiopathic, associated with systemic diseases or result from a variety of infectious agents. An annual estimated 17.6% of active uveitis patients experience transient or permanent loss of vision. Uveitis is responsible for more than 2.8% of cases of blindness in the U.S., making this disorder an important cause of vision loss and impairment. Non-infectious anterior uveitis is a debilitating form of intraocular inflammation of the anterior portion of the uvea, such as the iris and/or ciliary body and is the most common form of uveitis. Incidence in the U.S. ranges from approximately 26.6 to 102 per 100,000 adults annually with recent reports indicating occurrence in all age groups with the highest incidence in those over age 65 years. Chronic or recurrent, anterior uveitis may lead to complications such as posterior subcapsular cataract, glaucoma and macular edema.
Inflammation can be classified as either acute or chronic. Acute inflammation is the initial response of the body to harmful stimuli and is achieved by the increased movement of plasma and white blood cells from the blood into the injured tissues, in this case the uvea. Sometimes, the inflammation associated with anterior uveitis is in response to a real infection. This is known as infectious anterior uveitis. However, anterior uveitis often occurs for no apparent reason as the result of the immune system malfunctioning and triggering the process of inflammation even though no infection is present. This is known as non-infectious anterior uveitis. Patients that have anterior uveitis exhibit a large number of white blood cells in the anterior chamber of the eye. In order to count these cells in the anterior chamber, the physician uses a slit lamp, an instrument consisting of a high-intensity light source that can be focused to shine a thin sheet of light into the eye. The treatment objective is to eliminate the inflammation of the uvea which can be confirmed by an anterior chamber cell count of zero.
Clinical Trial Results
EyeGate OBG: Large Corneal Epithelial Defects
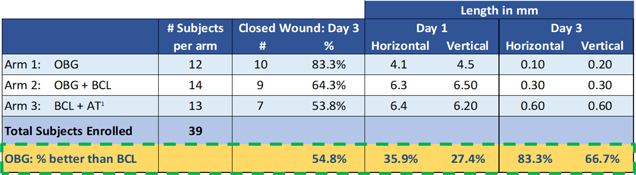
In the first quarter of 2017, we reported topline results from the first-in-human pilot trial of EyeGate OBG, the acceleration of re-epithelialization of large corneal epithelial defects in patients having undergone photorefractive keratectomy (“PRK”). The prospective, randomized, controlled study enrolled 39 subjects undergoing bilateral PRK surgery and aimed to assess the safety and performance of EyeGate OBG on its own or combined with a Bandage Contact Lens (“BCL”) compared to the current standard of care, artificial tears and BCL. The primary endpoint of the study was complete wound closure by Day 3.
The enrolled subjects were randomized into one of three study groups, with subjects receiving the same treatment in both eyes:
| · | Patients in arm 1 (n=12) received EyeGate Ocular Bandage Gel four times daily (QID) for two weeks after surgery. |
| · | Arm 2 (n=14) was comprised of EyeGate Ocular Bandage Gel QID for 2 weeks after surgery in combination with a BCL. |
| · | Arm 3 (n=13) was comprised of artificial tears administered four times daily and BCL. |
The study demonstrated safety and tolerability of EyeGate OBG, with encouraging potential efficacy. 83.3% of the subjects in Arm 1 (EyeGate OBG alone) achieved complete wound closure by Day 3, compared to 53.8% of patients that received the standard of care. Thus, the OBG arm had approximately 55% more subjects achieve full wound closure on Day 3 than the standard of care arm. Also, on Day 3, the average wound length, measured horizontally and vertically was 83.3% and 66.7% smaller, respectively, for the OBG arm versus the standard or care arm. Additionally, on Day 1 (24 hours post-surgery), the average wound length, measured horizontally and vertically, was 35.9% and 27.4% smaller, respectively, for the OBG arm versus the standard-of-care arm. Based on these positive results, EyeGate plans to continue development with a double-masked, controlled trial evaluating EyeGate OBG monotherapy against BCL in the first half of 2018.
| 6 |
EGP-437
We submitted an IND for EGP-437 to the FDA on April 28, 2008. The initial protocol submitted as part of the IND application was for our Phase 1/2 non-infectious anterior uveitis trial. Subsequently, we submitted amendments to our IND for protocols for additional trials that we have since completed on September 12, 2008, April 6, 2010, October 18, 2011, April 13, 2012 and May 20, 2015. An IND application (IND 107,846) referencing our IND (IND 77,888) was submitted by the University of Pennsylvania, School of Medicine on January 29, 2010 with a protocol for the treatment of anterior scleritis.
We have completed seven clinical trials under IND 107,846 for the EGP-437 Combination Product. The first two trials were executed in parallel - a Phase 1/2 non-infectious anterior uveitis trial and a Phase 2 dry eye trial. These two trials were followed by a Phase 3 dry eye trial. Subsequently, we completed our first Phase 3 trial for non-infectious anterior uveitis. During the time that we executed the Phase 3 non-infectious anterior uveitis trial we completed a Phase 2 proof-of-concept cataract surgery trial, with prophylactic treatment of the EGP-437 Combination Product. In 2016, we completed a Phase 1b/2a dose ranging trial treating inflammation and pain for subjects that have undergone cataract surgery and a Phase 1b/2a proof-of-concept macular edema trial.
| PROTOCOL | INDICATION | PHASE | NO. SUBJECTS RANDOMIZED | CONTROL ARM | ||||
| EGP-437-001 | Anterior Uveitis | 1/2 | 40 | None | ||||
| EGP-437-002 | Dry Eye | 2 | 105 | Placebo | ||||
| EGP-437-003 | Dry Eye | 3 | 198 | Placebo | ||||
| EGP-437-004 | Anterior Uveitis | 3 | 193 | Standard of care | ||||
| EGP-437-005 | Cataract Surgery | 2 POC | 45 | Placebo | ||||
| EGP-437-007 | Macular Edema | 1b/2a | 26 | None | ||||
| EGP-437-008 | Cataract Surgery | 1b/2a | 80 | Placebo |
Cataract Surgery: Phase 1b/2a Trial (EGP-437-008)
We have reported positive data for our dose-ranging clinical trial for the treatment of ocular inflammation and pain in post-surgical cataract patients. The Phase 1b/2a clinical trial was a multi-center, open-label trial enrolling 80 subjects who had undergone unilateral cataract extraction and implantation of a monofocal intra-ocular lens. The primary objective of this trial was to assess the safety and efficacy of iontophoretic EGP-437 in these patients following surgery.
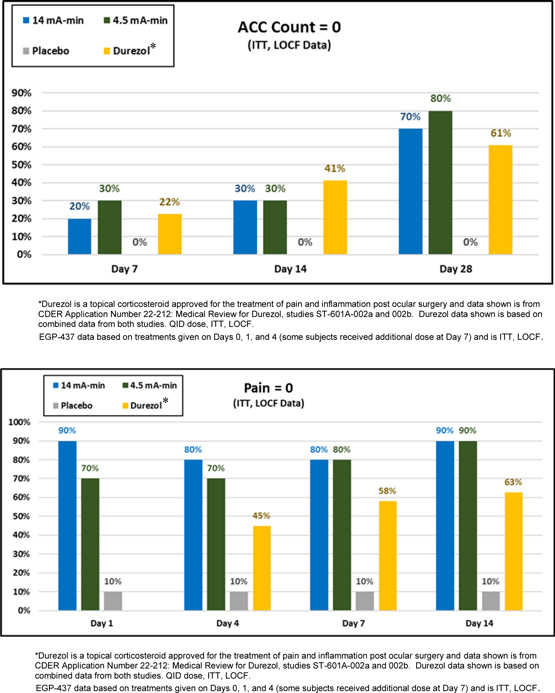
The trial design included eight cohorts, ten subjects per cohort, whereby iontophoretic doses of 4.0 mA-min, 4.5 mA-min, 9.0 mA-min and 14.0 mA-min were employed and the 9.0 and 14.0 mA-min cohorts included different dosing regimens. Dosing regimens included three treatments administered on Day 0, Day 1 and Day 2 or Day 0, Day 1 and Day 4 with potential for an additional treatment at Day 7 in all cohorts. One cohort had the Day 0 treatment given prior to surgery and all other cohorts had the Day 0 treatment provided after surgery. All cohorts except one was treatment delivering EGP-437, the exception was a placebo arm. The primary endpoint for all cohorts is based on the proportion of subjects that achieved an anterior chamber cell (ACC) count of zero, with secondary endpoints measuring pain score and intra-ocular pressure.
A positive response was achieved demonstrating that EGP-437 delivered via our EyeGate® II Delivery System was safe and effective in reducing inflammation and preventing pain. The best responses were achieved with the 4.5mA-min and 9.0mA-min cohorts with similar or greater percentage of patients with ACC count of zero greater than Durezol* at Day 7. Both EGP-437 cohorts demonstrated a greater proportion of patients with ACC count of zero than Durezol* at Day 28. The percentage of patients with zero pain was better than Durezol* at Day 4, 7 and 14 for both EGP-437 cohorts. The optimal dose was determined to take forward into a Phase 2b trial, initiated in the third quarter of 2017.
| 7 |
| 8 |
Cataract Surgery: Phase 2b Trial (EGP-437-009)
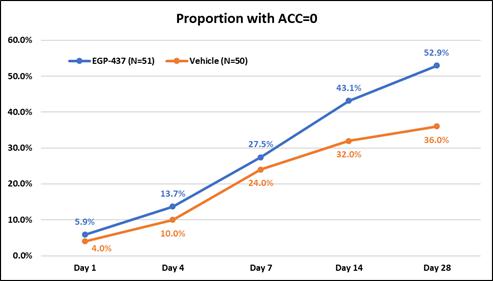
We announced topline data for this trial in the first quarter of 2018. Although EGP-437 demonstrated a higher rate of success compared to vehicle at all time points, the co-primary endpoints of proportion of subjects with an anterior chamber cell (ACC) count of zero at day 7 and the proportion of subjects with a pain score of zero at day 1 did not show statistical significance. The efficacy results for the absence of inflammatory cells in the EGP-437 treatment group met our expectations, but the vehicle group response was better than anticipated. The difference in proportion of subjects with total clearing of ACC between the EGP-437 group and the Placebo widens at Day 14 and Day 28, trending towards statistical significance (see graph below). Also, the difference in average or mean cell count at Day 7 (the day for evaluating the primary endpoint) between the EGP-437 group and the Placebo group was statistically significant with a P value = 0.0096.
We will continue to review the data to determine next steps and to continue evaluating EGP-437 for the reduction of inflammation and pain following ocular surgery.
| 9 |
Macular Edema: Phase 1b/2a Trial (EGP-437-007)
We have reported data for our first clinical trial treating a back of the eye indication, macular edema. The Phase 1b/2a proof-of-concept trial suggests that iontophoresis can non-invasively deliver EGP-437 to the back of the eye. The non-invasive delivery of EGP-437 has demonstrated a positive response in some patients with macular edema.
The completed Phase 1b / 2a clinical trial is a multi-center, open-label trial. The data reported was based on the first 19 patients enrolled and had macular edema associated with Retinal Vein Occlusion, Diabetic Retinopathy or Post-Surgical (cystoid) Macular Edema. The primary objective of this trial is to evaluate the safety and efficacy of iontophoretic EGP-437 in patients suffering from Macular Edema. Three treatments at 14.0 mA-min (3.5mA) were administered on Day 0, Day 4 and Day 9. Primary outcome of the trial measured reduction in mean central subfield thickness on Day 4, Day, 9 and Day 14. Ozurdex® was administered as control to patients that did not respond to the investigational therapy at Day 14 and were re-evaluated at Day 28.
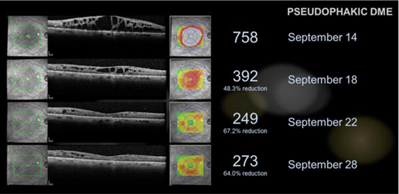
A positive response was observed in some of the patients, with pseudophakic eyes (an eye implanted with an intraocular lens) responding better than phakic eyes (an eye with a natural lens). A positive response was demonstrated in three subpopulations of macular edema including macular edema associated with diabetes, retinal vein occlusion and inflammation or cystoid. In one example, a subject that presented with diabetic macular edema was provided with three treatments of EGP-437, Day 0, Day 4 and Day 9 and showed anatomic resolution in approximately one week after only two treatments, as illustrated by the optical coherence tomography scan below. Additionally, the investigational therapy showed no serious treatment emergent adverse effects including no increase in ocular pressure even at three times the iontophoretic dose that was used for the Company’s Phase 3 non-infectious anterior uveitis trial.
Non-Infectious Anterior Uveitis: Phase 3 Clinical Trial (EGP-437-004)
Our previous Phase 1/2 non-infectious anterior uveitis clinical trial, and two dry eye clinical trials, showed that the EGP-437 dose selected for the Phase 3 non-infectious anterior uveitis trial was well tolerated and demonstrated positive activity. The Phase 3 non-infectious anterior uveitis clinical trial was conducted to assess safety and efficacy of the EGP-437 Combination Product and evaluate its non-inferiority status to a standard of care, prednisolone acetate 1% (PA) eye drops. Communication received from the FDA, dated December 3, 2007, stated that the FDA recommends that PA, administered at least four times per day (q.i.d.), be the positive control agent for the treatment of anterior uveitis. Our trial utilized a more stringent regimen for the positive control of eight times per day in week one and six times per day in week two before going to four times per day in weeks three and four. Patients had to agree to comply with dosing regimen to be included in the trial.
The completed Phase 3 non-inferiority study in patients with non-infectious anterior uveitis appeared to demonstrate that two iontophoretic treatments with our EGP-437 Combination Product achieved the same response rate as the positive control for the primary efficacy endpoint, a complete clearing of anterior chamber cells, by Day 14. The control is the current standard of care, PA, which was administered multiple times daily as eye drops. Although we achieved the same response rate in our Phase 3 clinical trial, the dose of the EGP-437 Combination Product tested was just outside the pre-set non-inferiority margin for intent-to-treat and per protocol populations and did not achieve statistical significance in the intent-to-treat population as compared to the positive control based on the primary efficacy endpoint.
| · | The EGP-437 Combination Product produced the same outcomes compared to PA while eliminating the need to apply up to eight eye drops a day, for a total of 154 drops over a four-week period - eight times per day for week one, six times per day for week two and four times per day for weeks three and four. |
| · | This was achieved with a lower incidence of increased IOP, which is characterized as an increase of six mm Hg or more from baseline; in the EGP-437 Combined Product group, 14 subjects had 17 occurrences while 24 subjects had 41 occurrences in the PA arm. |
| 10 |
Phase 3 Safety Discussion
Our EGP-437 Combination Product appears to be clinically comparable to PA topical drops. With regard to elevated IOP, no subjects in the EGP-437 Combination Product treatment arm experienced any significant increase in IOP (greater than 20mmHg), whereas the PA treatment arm had one subject with a reported IOP increase of 27mmHg. With regard to IOP-related adverse events, one subject in the EGP-437 Combination Product treatment group reported an adverse event (seen approximately three weeks after rescue was initiated) and six subjects in the PA treatment arm reported adverse events related to IOP.
Phase 3 Clinical Trial Conclusion
Topical corticosteroid therapy administered as frequently as every hour with tapering over the treatment period has been the mainstay for uveitis treatment since the 1950s. In this unique Phase 3 randomized, double-masked, positive-controlled clinical trial in subjects with non-infectious anterior uveitis, two treatments with ocular iontophoretic delivery of EGP-437 appears to be clinically comparable to PA topical drops administered with a tapering schedule from eight drops per day to four drops per day over 28 days.
By Days 7 and 14, the proportion of subjects reaching ACC counts of zero was slightly greater in the EGP-437 Combination Product arm than the PA arm. This effect was more noticeable in the subgroup of subjects with a higher baseline ACC count; a higher proportion of subjects in the EGP-437 Combination Product arm reached an ACC count of zero by Days 7 and 14 in this sub-group of subjects. Safety findings were comparable for both study arms.
Non-Infectious Anterior Uveitis: Phase 1/2 Trial (EGP-437-001)
Our first clinical trial initiated with the EGP-437 Combination Product was a Phase 1/2 trial for subjects with non-infectious anterior uveitis, which was defined as having anterior chamber cell (ACC) scores ≥ 1.5, or in other words, cell counts of less than or equal to 11 cells. Subjects who have anterior uveitis, exhibit a large number of white blood cells in the anterior chamber of the eye. The treatment objective is to eliminate the inflammation which can be visually confirmed when all white blood cells have been cleared from the anterior chamber. The degree of intraocular inflammation is based on a grading scheme or score that uses an ordinal scale ranging from 0 to 4.
The primary objective of this exploratory study was to define a safe and effective dose of EGP-437 in subjects with non-infectious anterior segment uveitis. The secondary objective was to evaluate the systemic pharmacokinetic profile of EGP-437 (dexamethasone and dexamethasone phosphate) following ocular dosing.
This multi-site, randomized, double-masked, parallel group, dose comparison, exploratory study comprised five visits conducted over 28 days. The study population was comprised of 40 eyes of 40 subjects. Enrolled subjects were randomly assigned to receive one of four iontophoresis dose levels of EGP-437 for approximately four minutes with up to ten subjects per treatment arm. Subjects received a single treatment only, at Day 0, subjects returned for examination on Days 1, 7, 14, and 28. Eligible subjects received one of the following four iontophoresis dose levels of EGP-437 (dexamethasone phosphate ophthalmic solution (40mg/mL)) for approximately 4 minutes:
| · | Treatment Group A: 1.6 mA-min at 0.4 mA |
| · | Treatment Group B: 4.8 mA-min at 1.2 mA |
| · | Treatment Group C: 10.0 mA-min at 2.5 mA |
| · | Treatment Group D: 14.0 mA-min at 3.5 mA |
Following the single treatment with the EGP-437 Combination Product, 48% of the subjects achieved an ACC score of zero within two weeks. By Day 28, 60% of the subjects achieved an ACC score of zero and required no further treatment. At Day 14, in the lowest treatment group, the proportion of subjects with an ACC count of zero was 4/10 (40%) and for all treatment groups was 7/40 (18%). At Day 28, in the lowest treatment group, the proportion of subjects with an ACC count of zero was higher at 6/10 (60%) and for all treatment groups was 14/40 (35%). The highest proportion of subjects with an ACC score or ACC count of zero was in the 1.6 mA-min at 0.4 mA treatment group at both Days 14 and 28.
| TREATMENT GROUP | ||||||||||||||||||||||
| CHARACTERISTIC | STATISTIC OR CATEGORY | 1.6 mA-min (N = 10) |
4.8 mA-min (N = 10) |
10.0 mA-min (N = 10) |
14.0 mA-min (N = 10) |
Total (N = 40) |
||||||||||||||||
| ACC Score of Zero | Day 14 | 8 (80 | )% | 6 (60 | )% | 2 (20 | )% | 3 (30 | )% | 19 (48 | )% | |||||||||||
| Day 28 | 8 (80 | )% | 6 (60 | )% | 5 (50 | )% | 5 (50 | )% | 24 (60 | )% | ||||||||||||
| ACC Count of Zero | Day 14 | 4(40 | )% | 1 (10 | )% | 1 (10 | )% | 1 (10 | )% | 7 (18 | )% | |||||||||||
| Day 28 | 6 (60 | )% | 2 (20 | )% | 1 (10 | )% | 5 (50 | )% | 14 (35 | )% | ||||||||||||
| 11 |
The median time in days to an ACC score of zero ranged from a minimum of 11.5 days in the 1.6 mA-min dose group to a maximum of 31.0 days in the 14.0 mA-min dose group. The proportion of patients with an ACC score reduction of 0.5 or more on Day 28 was 80% (eight) in the 1.6 mA-min dose group and 60% (six) in the other three dose groups. The mean change in ACC score from baseline to Day 28 ranged from a maximum of -2.25 in the 1.6 mA-min dose group to a minimum of -2.00 in the 14.0 mA-min dose group. The relatively short mean times to reach an ACC score of zero in each dose group suggest that the treatment has a rapid onset of action.
The results from this trial appeared to demonstrate that the most effective EGP-437 dose level is in the 1.6 mA-min at 0.4 mA dose level. The level of association between the iontophoresis treatments and achieving an ACC Score of zero was assessed and the association was estimated to be statistically significant at a 5% level of significance (p-value = 0.032) on Day 14, suggesting that the treatment differences are larger than would be expected by chance alone. The probability-value or p-value is a number between 0.00 and 1.00, and is used to demonstrate the strength of a conclusion drawn from clinical trial data. Essentially the p-value measures consistency between the results actually obtained in the trial and the “pure chance” explanation for those results. A statement and corresponding p-value are considered of strong significance if the probability of the same reaction occurring randomly or by chance is less than 5%, corresponding to a p-value of p<0.05.
This trial showed low short-term systemic exposure to dexamethasone following ocular iontophoresis delivery of dexamethasone phosphate, and no corticosteroid mediated effects were observed.
While this dose-ranging study did not include positive or negative controls, the results demonstrated that a single treatment with the EGP-437 Combination Product: (1) lowered ACC scores in the majority of patients without requiring additional treatment and (2) produced low short-term systemic exposure to dexamethasone and dexamethasone phosphate.
Clinical Development Plan
EyeGate OBG
The EyeGate OBG has been shown to provide a mechanical barrier that aids in the management of corneal epithelial wounds, defects and epitheliopathies. EyeGate OBG has been shown to accelerate re-epithelization in both preclinical studies and in clinical ophthalmic veterinary use. As such, photorefractive keratectomy (PRK) surgery was chosen as the subject population, which is best suited to demonstrate the acceleration of re-epithelization. PRK is an efficacious alternative to patients seeking surgical correction of refractive errors who are not suitable candidates for laser in situ keratomileusis (LASIK) due to inadequate corneal thickness, larger pupil size, history of keratoconjunctivitis sicca (KCS), or anterior basement membrane disease. The primary effectiveness endpoint for this initial pilot trial was time to re-epithelization of epithelial defect following PRK surgery. We have completed the initial proof-of-concept trial and announced positive top-line data in the first quarter of 2017. We anticipate initiating a prospective, masked pilot clinical trial in the first half of 2018 for large corneal epithelial defects following PRK surgery.
The FDA, at the pre-submission meeting that occurred in the fourth quarter of 2016, asked us to file an Investigational Device Exemption (IDE) application prior to continuing with the development of OBG. The IDE was filed in May of 2017 and in June of 2017, 30 days following submission, we received a comment letter from the FDA. The letter asked us to complete specific tasks and to submit an IDE amendment with the results for those tasks. The majority of the comments were related to the validation of the manufacturing process for OBG. Due to the chemical characteristics of OBG, we are unable to terminally sterilize our final product. Terminal sterilization means that the product in its final container is subjected to a sterilization process such as heat or irradiation. We provide a sterile product produced by aseptic processing. In an aseptic process, the drug product, container, and closure are first subjected to sterilization methods separately, as appropriate, and then brought together. Because there is no process to sterilize the product in its final container, it is critical that containers be filled and sealed in an extremely high-quality environment. Aseptic processing involves more variables than terminal sterilization. Before aseptic assembly into a final product, the individual parts of the final product are generally subjected to various sterilization processes. Each of these manufacturing processes requires validation and control. A terminally sterilized product, on the other hand, undergoes final sterilization in a sealed container, thus limiting the possibility of error.
Some of the items requested from the FDA included tasks such as:
| · | Evaluate the manufacturing process to eliminate sources which could contribute to excessive bioburden levels, |
| · | Provide alert and action levels for device components prior to filter sterilization, |
| · | Provide description of validation protocol and bacterial retention results for sterilizing grade filters, |
| · | Provide percent recovery results for bioburden test methods, |
| · | Validate gamma irradiation dose for device packaging, and |
| · | Include validated analytical methods to identify and quantify impurities. |
We plan on filing the IDE amendment in the first quarter of 2018 and anticipate commencing our next PRK trial in the first half of 2018. We also plan on submitting a second IDE in the first quarter of 2018 for the development of OBG for the treatment of Punctate Epitheliopathies, including dry eye. We anticipate commencing this pilot trial in the first half of 2018.
| 12 |
EGP-437: Cataract Surgery
We have completed three trials (Phase 2 prophylactic, Phase 1b/2a dose-ranging and Phase 2b) and reported positive data for our Phase 1b/2a dose-ranging clinical trial for the treatment of ocular inflammation and pain in post-surgical cataract patients. The design of this trial is based on treating the patients’ post-surgery and not prophylactically. The Phase 1b/2a clinical trial was a multi-center, open-label trial enrolling 80 subjects who had undergone unilateral cataract extraction and implantation of a monofocal intra-ocular lens. The primary objective of this trial was to assess the safety and efficacy of iontophoretic EGP-437 in these patients following surgery. A positive response was achieved and an optimal dose was determined to take forward into a Phase 2b trial that was initiated in the third quarter of 2017. We announced topline data for the Phase 2b trial in the first quarter of 2018. Although EGP-437 demonstrated a higher rate of success compared to vehicle at all time points, the co-primary endpoints of proportion of subjects with an anterior chamber cell (ACC) count of zero at day 7 and the proportion of subjects with a pain score of zero at day 1 did not show statistical significance. The efficacy results for the absence of inflammatory cells in the EGP-437 treatment group met our expectations, but the vehicle group response was better than anticipated. We will continue to review the data to determine next steps and to continue evaluating EGP-437 for the reduction of pain and inflammation following ocular surgery.
EGP-437: Anterior Uveitis
We have completed two trials (Phase 1/2 and Phase 3) for anterior uveitis and have demonstrated in the completed Phase 3 non-inferiority study that two iontophoretic treatments with our EGP-437 Combination Product achieved the same response rate as the positive control for the primary efficacy endpoint, a complete clearing of anterior chamber cells, by Day 14. This was achieved with a lower incidence of increased IOP, which is characterized as an increase of six mm Hg or more from baseline. We currently have an ongoing confirmatory Phase 3 trial underway and anticipate top-line data in the second quarter of 2018. The FDA has provided guidance that the ongoing confirmatory Phase 3 trial of EGP-437 in anterior uveitis meets non-inferiority criteria, data from this trial along with data from our previously completed Phase 3 trial in anterior uveitis will be sufficient to support an NDA filing. The FDA also communicated that the design of the ongoing confirmatory Phase 3 anterior uveitis trial is acceptable and that the nonclinical work completed to date is sufficient to support an NDA filing.
EGP-437: Other Indications
Although we have completed two trials (Phase 2 and Phase 3) for dry eye, at this time we are not anticipating any further development for this indication. We have completed a Phase 1/2 for macular edema and at this time we are assessing the next steps for this indication.
Intellectual Property and Proprietary Rights
Overview
We are building an intellectual property portfolio for our EGP-437 Combination Product and CMHA-S platform, as well as other devices and product candidates for treatment of ocular indications in the U.S. and abroad. We currently seek, and intend to continue to seek, patent protection in the U.S. and internationally for our product candidates, methods of use, and processes for manufacture, and for other technologies, where appropriate. Our current policy is to actively seek to protect our proprietary position by, among other things, filing patent applications in the U.S. and abroad relating to proprietary technologies that are important to the development of our business. We also rely on, and will continue to rely on, trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our technology.
Our success will depend significantly on our ability to obtain and maintain patent and other proprietary protection for the technologies that we consider important to our business, our ability to defend our patents, and our ability to preserve the confidentiality of our trade secrets and operate our business without infringing the patents and proprietary rights of third parties.
Patent Portfolio
Our patent portfolio includes drug delivery device patents directed to the EyeGate® II Delivery System, drug composition patents directed to EGP-437 and other compositions and devices related to the EyeGate® II Delivery System. In addition, further patent applications are directed to the CMHA-S platform in combination with active therapeutics to treat ocular diseases. These issued patents will expire between 2018 and 2034.
We have been developing drug compositions and drug delivery systems for non-invasive treatment on the eye for several years. These delivery systems include various patented and patent pending drug delivery devices, active therapeutics and combination device/therapeutic to treat components of the eye, such as the cornea, sclera, and combinations thereof. These devices and therapeutics have been further improved to provide better patient comfort levels, patient compliance and recovery times. The delivery system patent portfolio consists of seven Patent families, which includes fifteen U.S. Patents and 87 corresponding International Patents. We hold fifteen patents (thirteen issued and two allowed). Additionally, we hold 103 patents by way of our subsidiary, EyeGate Pharma S.A.S., a French corporation, or EyeGate S.A.S.
| 13 |
License Agreements
We are a party to six license agreements as described below. Four of the six license agreements require us to pay royalties or fees to the licensor based on revenue related to the licensed technology, and the agreements with Valeant require Valeant to pay royalties to us based on revenue related to the licensed technology.
On February 15, 1999, we entered in to an exclusive worldwide license agreement with the University of Miami School of Medicine to license technology relating to our EyeGate® II Delivery System, which grants us the right to use certain French, European, Canadian, Japanese, American, Mexican, Korean, Brazilian and Israeli patents in our EGP-437 Combination Product. This agreement, which was amended in December 2005, requires us to pay to the University of Miami an annual license fee of $12,500. This license also requires payments to the University of Miami upon our achievement of certain milestones. All annual license fee and milestone payments have been paid to date. The total amount of milestone payments paid to date under this license agreement is $30,000 and there are potential aggregate additional amounts of up to $70,000 due on certain milestones being met. This license agreement remains in effect until the later of twelve (12) years after the date of the first commercial sale of the applicable product or the date of the last to expire patent relating to the patent rights under the Agreement. Upon such expiration and assuming it was not terminated earlier in accordance with its terms, we retain a fully paid up and perpetual license to the product and certain intellectual property. The license agreement also provides that it may be terminated by either party in the case of continued material breach or provision of false reports, by the licensor pertaining to certain bankruptcy or insolvency circumstances regarding our company or by us upon ninety (90) days prior written notice.
On July 23, 1999, we entered into a perpetual Transaction Protocol agreement with Francine Behar-Cohen to acknowledge our right to use certain patents that Ms. Behar-Cohen had certain ownership rights with respect to and which are used in our EGP-437 Combination Product. The agreement also provides for us to pay Ms. Behar-Cohen a fee based on a percentage of the pre-tax turnover generated from sales of our EGP-437 Combination Product relating to our inclusion of the EyeGate® II Delivery System. The fees due under the agreement expired in January 2018.
On September 12, 2013, Jade entered into an agreement with BioTime, Inc. granting to it the exclusive worldwide right to commercialize cross-linked thiolated carboxymethyl hyaluronic acid (“CMHA-S”) for ophthalmic treatments in humans. The agreement calls for a license issue fee paid to BioTime of $50,000, and requires us (through our Jade subsidiary) to pay an annual fee of $30,000 and royalties to BioTime based on revenue relating to any product incorporating the CMHA-S technology. The agreement expires when patent protection for the CMHA-S technology lapses.
On July 9, 2015, we entered into an exclusive worldwide licensing agreement with a subsidiary of Valeant through which we have granted Valeant exclusive, worldwide commercial and manufacturing rights to our EGP-437 Product in the field of anterior uveitis, as well as a right of last negotiation to license the EGP-437 Product for other indications. Under the agreement, Valeant paid us an upfront payment of $1.0 million. We are eligible to receive milestone payments totaling up to $32.5 million, upon and subject to the achievement of certain specified developmental and commercial milestones. In addition, we are eligible to receive royalties based on a specified percent of net sales of the Product throughout the world, subject to adjustment in certain circumstances.
On June 17, 2016, we entered into an exclusive worldwide license agreement with the University of Utah Research Foundation to further the commercial development of the NASH technology, together with alkylated HA. The agreement calls for payments due to the University of Utah, consisting of a license grant fee of $15,000 due within 30 days of signing, and an annual licensing fee, initially $5,000, and escalating ratably up to $20,000 in 2021.
On February 21, 2017, we entered into an exclusive, worldwide licensing agreement with a subsidiary of Valeant (the “New Valeant Agreement”), through which we granted Valeant exclusive, worldwide commercial and manufacturing rights to its EGP-437 Product in the field of ocular iontophoretic treatment for post-operative ocular inflammation and pain in ocular surgery patients (the “New Field”). Under the New Valeant Agreement, Valeant paid us an initial upfront payment of $4.0 million, and we are eligible to receive milestone payments totaling up to approximately $99.0 million, upon and subject to the achievement of certain specified developmental and commercial progress of the EGP-437 Product for the New Field. In addition, we are eligible under the New Valeant Agreement to receive royalties based on a specified percent of net sales of its EGP-437 Product for the New Field throughout the world, subject to adjustment in certain circumstances.
Confidential Information and Inventions Assignment Agreements
We currently require and will continue to require each of our employees and consultants to execute confidentiality agreements upon the commencement of such individual’s employment, consulting or collaborative relationships with us. These agreements provide that all confidential information developed or made known during the course of the relationship with us be kept confidential and not disclosed to third parties except in specific circumstances.
In the case of employees, the agreements provide that all inventions resulting from such individual’s work performed for us, utilizing our property or relating to our business and conceived or completed by the individual during employment shall be our exclusive property to the extent permitted by applicable law. Our consulting agreements also provide for assignment to us of any intellectual property resulting from services performed by a consultant for us.
| 14 |
Sales and Marketing
We have entered into two exclusive global License Agreements with subsidiaries of Valeant Pharmaceuticals International, Inc. (“Valeant”), through which we have granted Valeant exclusive, worldwide commercial and manufacturing rights to its EyeGate® II Delivery System and EGP-437 combination product (“Product”) in the fields of anterior uveitis and ocular iontophoretic treatment for post-operative ocular inflammation and pain in ocular surgery patients, as well as a right of last negotiation to license the Product for other indications.
If EyeGate OBG is approved by the FDA for commercial sale, we may enter into agreements with third parties to sell EyeGate OBG or we may choose to market EyeGate OBG directly to physicians in the United States through our own sales and marketing force and related internal commercialization infrastructure. If we market EyeGate OBG directly, we will need to incur significant additional expenses and commit significant additional management resources to establish and train an internal sales and marketing force to market and sell EyeGate OBG.
Manufacturing
We do not have, and do not intend to establish an in-house manufacturing capability for our products and as a result we will depend heavily on third-party contract manufacturers to produce and package our products. We currently do not have any contractual relationships with third-party manufacturers. We intend to rely on third-party suppliers that we have used in the past for the manufacturing of various components that comprise our EGP-437 Combination Product, EyeGate OBG and other contemplated clinical trials.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technologies, knowledge, experience and scientific resources provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
Our potential competitors include large pharmaceutical and biotechnology companies, and specialty pharmaceutical and generic drug companies. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
The key competitive factors affecting the success of each of our product candidates, if approved for marketing, are likely to be its efficacy, safety, method of administration, convenience, price, the level of generic competition and the availability of coverage and adequate reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors’ establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third party payors seeking to encourage the use of generic products. Generic products currently being used for the indications that we may pursue, and additional products are expected to become available on a generic basis over the coming years. If our product candidates achieve marketing approval, we expect that they will be priced at a significant premium over competitive generic products.
| 15 |
Our competitors in the treatment of non-infectious anterior uveitis and inflammation post cataract surgery include Durezol® (Novartis AG), Lotemax® (Valeant Pharmaceuticals International, Inc.), Pred Forte® (Allergan, Inc.) and prednisolone acetate ophthalmic suspension (1%) (Novartis AG). We are not aware of any FDA approved eye drops for the management and the acceleration of re-epithelization of corneal epithelial defects following photorefractive keratectomy (PRK) surgery.
Government Regulation
FDA Approval Process
In the U.S., pharmaceutical products are subject to extensive regulation by the FDA. The Food Drug and Cosmetic Act, or FDCA, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable FDA or other requirements may subject a company to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending applications, a clinical hold, warning letters, recall or seizure of products, partial or total suspension of production, withdrawal of the product from the market, injunctions, fines, civil penalties or criminal prosecution.
FDA approval is required before any new drug, can be marketed in the U.S. The process required by the FDA before a new drug product may be marketed in the U.S. generally involves:
| · | completion of preclinical laboratory and animal testing and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulation; |
| · | submission to the FDA of an IND for human clinical testing which must become effective before human clinical trials may begin in the U.S.; |
| · | approval by an independent institutional review board, or IRB, at each site where a clinical trial will be performed before the trial may be initiated at that site; |
| · | performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, to establish the safety and efficacy of the proposed product candidate for each intended use; |
| · | satisfactory completion of an FDA pre-approval inspection of the facility or facilities at which the product is manufactured to assess compliance with the FDA’s cGMP regulations; |
| · | submission to the FDA of a new drug application, or NDA, which must be accepted for filing by the FDA; |
| · | satisfactory completion of an FDA advisory committee review, if applicable; |
| · | payment of user fees, if applicable; and |
| · | FDA review and approval of the NDA. |
| 16 |
The preclinical and clinical testing and approval process requires substantial time, effort and financial resources. Pre-clinical tests include laboratory evaluation of product chemistry, formulation, manufacturing and control procedures and stability, as well as animal studies to assess the toxicity and other safety characteristics of the product. The results of preclinical tests, together with manufacturing information, analytical data and a proposed clinical trial protocol and other information, are submitted as part of an IND to the FDA. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions and places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. A clinical hold may occur at any time during the life of an IND and may affect one or more specific studies or all studies conducted under the IND.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with GCPs. They must be conducted under protocols detailing the objectives of the trial, dosing procedures, research subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND, and progress reports detailing the results of the clinical trials must be submitted at least annually. In addition, timely safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. An institutional review board, or IRB, at each institution participating in the clinical trial must review and approve each protocol before a clinical trial commences at that institution and must also approve the information regarding the trial and the consent form that must be provided to each trial subject or his or her legal representative, monitor the study until completed and otherwise comply with IRB regulations.
Sponsors of clinical trials generally must register and report, at the NIH-maintained website ClinicalTrials.gov, key parameters of certain clinical trials. For purposes of an NDA submission and approval, human clinical trials are typically conducted in the following sequential phases, which may overlap or be combined:
| · | Phase 1: The product is initially introduced into healthy human patients and tested for safety, dose tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain an early indication of its effectiveness. |
| · | Phase 2: The product is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted indications and to determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more extensive clinical trials. |
| · | Phase 3: These are commonly referred to as pivotal studies. When Phase 2 evaluations demonstrate that a dose range of the product appears to be effective and has an acceptable safety profile, trials are undertaken in large patient populations to further evaluate dosage, to obtain additional evidence of clinical efficacy and safety in an expanded patient population at multiple, geographically-dispersed clinical trial sites, to establish the overall risk-benefit relationship of the product and to provide adequate information for the labeling of the product. |
| · | Phase 4: In some cases, the FDA may condition approval of an NDA for a product candidate on the sponsor’s agreement to conduct additional clinical trials to further assess the product’s safety and effectiveness after NDA approval. Such post-approval trials are typically referred to as Phase 4 studies. |
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling, and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA is subject to the payment of user fees; a waiver of such fees may be obtained under certain limited circumstances. The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review.
| 17 |
Section 505(b)(2) New Drug Applications
According to section 505 of the FDCA, there are three types of new drug applications: (1) an application that contains full reports of investigations of safety and effectiveness (section 505(b)(1)); (2) an application that contains full reports of investigations of safety and effectiveness but where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference from the entity that performed the studies (section 505(b)(2)); and (3) an application that contains information to show that the proposed product is identical in active ingredient, dosage form, strength, route of administration, labeling, quality, performance characteristics, and intended use, among other things, to a previously approved product (section 505(j)). We intend to submit a 505(b)(2) NDA for our EGP-437 Combination Product.
Section 505(b)(2) of the FDCA enables an applicant to rely, in part, on the FDA’s findings of safety and efficacy for an existing product, or published literature, in support of its NDA. Using this approval pathway may allow us to rely in part on information in the public domain to support the safety and effectiveness of EGP-437. The FDA may also require sponsors to perform additional clinical trials, measurements, or other types of studies or assessments (e.g., bridging studies) to support any change from the previously approved product. The review process is lengthy and often difficult, and the FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. The FDA may issue a complete response letter, which may require additional clinical or other data or impose other conditions that must be met in order to secure final approval of the NDA or an approved letter following satisfactory completion of all aspects of the review process. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured.
To the extent that a Section 505(b)(2) NDA relies on clinical trials conducted for a previously approved drug product or the FDA’s prior findings of safety and effectiveness for a previously approved drug product, the 505(b)(2) applicant must submit patent certifications in its 505(b)(2) application with respect to any patents listed for the approved product on which the application relies in the FDA’s publication, Approved Drug Products with Therapeutic Equivalence Evaluations (commonly referred to as the Orange Book). Specifically, the applicant must certify for each listed patent that (1) the required patent information has not been filed; (2) the listed patent has expired; (3) the listed patent has not expired, but will expire on a particular date and approval is not sought until after patent expiration; or (4) the listed patent is invalid, unenforceable or will not be infringed by the proposed new product. A certification that the new product will not infringe the previously approved product’s listed patent or that such patent is invalid or unenforceable is known as a Paragraph IV certification. If the applicant does not challenge one or more listed patents through a Paragraph IV certification, the FDA will not approve the Section 505(b)(2) NDA application until all the unchallenged listed patents claiming the referenced product have expired. Further, the FDA will also not accept or approve, as applicable, a Section 505(b)(2) NDA application until any non-patent exclusivity, such as exclusivity for obtaining approval of a New Chemical Entity, listed in the Orange Book for the referenced product, has expired.
| 18 |
If the 505(b)(2) NDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the owner of the referenced NDA for the previously approved product and relevant patent holders within 20 days after the 505(b)(2) NDA has been accepted for submission by the FDA. The NDA and patent holders may then initiate a patent infringement suit against the 505(b)(2) applicant. Under the FDCA, the filing of a patent infringement lawsuit within 45 days of receipt of the notification regarding a Paragraph IV certification automatically prevents the FDA from approving the Section 505(b)(2) NDA for 30 months, or until a court deems the patent unenforceable, invalid or not infringed, whichever is earlier. Moreover, in cases where a 505(b)(2) application containing a Paragraph IV certification is submitted during a previously approved drug’s five-year exclusivity period, the 30-month period is automatically extended to prevent approval of the 505(b)(2) application until the date that is seven and one-half years after approval of the previously approved reference product. The court also has the ability to shorten or lengthen either the 30 month or the seven and one-half year period if either party is found not to be reasonably cooperating in expediting the litigation. Thus, the Section 505(b)(2) applicant may invest a significant amount of time and expense in the development of its product only to be subject to significant delay and patent litigation before its product may be commercialized. Alternatively, if the NDA applicant or relevant patent holder does not file a patent infringement lawsuit within the specified 45-day period, the 30-month stay will not prevent approval of the 505(b)(2) application.
Notwithstanding the approval of many products by the FDA pursuant to Section 505(b)(2), over the last few years, some pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA changes its interpretation of Section 505(b)(2), or if the FDA’s interpretation is successfully challenged in court, this could delay or even prevent the FDA from approving any Section 505(b)(2) NDAs that we submit.
In the NDA submissions for our product candidates, we intend to follow the development and approval pathway permitted under the FDCA that we believe will maximize the commercial opportunities for these product candidates.
Combination Product Regulations
Medical products containing a combination of new drugs, biological products, or medical devices may be regulated as “combination products” in the U.S. A combination product generally is defined as a product comprised of components from two or more regulatory categories, such as drug/device, device/biologic, or drug/biologic. Each component of a combination product is subject to the requirements established by the FDA for that type of component, whether a new drug, biologic, or device. In order to facilitate premarket review of combination products, the FDA designates one of its centers to have primary jurisdiction for the premarket review and regulation of both components. We expect that the Center for Drug Evaluation and Research will have primary jurisdiction over out EGP-437 Combination Product. The determination whether a product is a combination product or two separate products is made by the FDA on a case-by-case basis. We have had discussions with the FDA about the status of our EGP-437 Combination Product as a combination product and we have been told that the FDA considers our product a combination drug/device.
We will be subject to regulations governing medical devices separate from those governing drugs. After the FDA permits a device to enter commercial distribution, however, numerous regulatory requirements apply. These include:
| · | product labeling regulations; |
| · | general prohibition against promoting products for unapproved or “off-label” uses; |
| 19 |
| · | corrections and removals (e.g., recalls); |
| · | establishment registration and device listing; |
| · | general prohibitions against the manufacture and distribution of adulterated and misbranded devices; and |
| · | the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. |
Failure to comply with these regulatory requirements could result in civil fines, product seizures, injunctions, and/or criminal prosecution of responsible individuals and us.
Approval or Clearance of Medical Devices
Medical devices, such as our EyeGate® II Delivery System, or the EyeGate® OBG, may be evaluated either through the premarket approval, or PMA process, or the 510(k) clearance process, depending on the classification of the device.
The regulatory classification for the EyeGate® II Delivery System is defined under Code of Federations Regulations 21, Part 890, section 5525 (21 CFR 890.5525). The FDA has confirmed that the EyeGate® II Delivery System will be submitted under the 510(k) clearance process. The FDA has further clarified the Code to state that an iontophoresis device intended for use with a specific drug that has been approved for delivery by iontophoresis is a class II device. The EyeGate® II Delivery System will be indicated for use with a specific drug (EGP-437) that will be approved through the NDA process and therefore classified as a class II device.
The FDA has confirmed that the EyeGate® OBG will be submitted under the 510(k) de novo clearance process when used as a standalone device.
Gathering clinical evidence for devices is subject to FDA’s good clinical practice regulations, including requirements for IRB approval and informed consent. Significant risk devices require an approved investigational device exemption application before studies may begin. PMA approval typically requires, among other things, the submission of valid scientific evidence in the form of preclinical and clinical data, and a pre-approval inspection to determine if the manufacturing facility complies with cGMP practices under the quality system regulation that governs the design and all elements of the manufacture of devices. For clearance, a 510(k) must demonstrate substantial equivalence, i.e., must show that the device is as safe and effective as an already legally marketed device, also known as a predicate device. The evaluation of the newer device must not raise different questions of safety and effectiveness than that of the predicate device. 510(k)s normally do not, but sometimes do, require clinical data for clearance.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to product/device listing, recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product.
| 20 |
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and generally require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| · | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| · | fines, warning letters or holds on post-approval clinical trials; |
| · | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; |
| · | product seizure or detention, or refusal to permit the import or export of products; or |
| · | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. While physicians may prescribe for off label uses, manufacturers may only promote for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off label uses, and a company that is found to have improperly promoted off label uses may be subject to significant liability, both at the federal and state levels.
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of the use of our drug candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and the application for extension must be made prior to expiration of the patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond their current expiration date, depending on the expected length of clinical trials and other factors involved in the submission of the relevant NDA.
| 21 |
Market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an approved NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Manufacturing Requirements
We and our third party manufacturers must comply with applicable FDA regulations relating to FDA’s cGMP regulations. The cGMP regulations include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, extensive records and reports, and returned or salvaged products. The manufacturing facilities for our products must meet cGMP requirements to the satisfaction of the FDA pursuant to a pre-approval inspection before we can use them to manufacture our products. We and our third party manufacturers and certain key component suppliers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including untitled letters, warning letters, determinations of product adulteration, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties. Adverse experiences with the product must be reported to the FDA and could result in the imposition of market restrictions through labeling changes or in product removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval. Such perceived problems concerning safety or efficacy may arise in the context of clinical studies continued as a result of our post-marketing obligations, reports we or FDA receive from patients and healthcare providers, or literature published by third parties regarding our products or similar products.
Third Party Payor Coverage and Reimbursement
Reimbursement is expected to use standard approaches for Ophthalmology with EGP-437 reimbursed as a physician-administered drug using a drug code (J-code) and the procedure reimbursed via a CPT code in addition to the standard reimbursement for office visits. The commercial success of our EGP-437 Combination Product and, if and when commercialized, our other product candidates will depend, in part, upon the availability of coverage and reimbursement from third party payors at the federal, state and private levels, including U.S. Government payor programs, such as Medicare and Medicaid, private health care insurance companies and managed care plans have attempted to control costs by limiting coverage and the amount of reimbursement for particular procedures or drug treatments. The U.S. Congress and state legislatures from time to time propose and adopt initiatives aimed at cost containment, which could impact our ability to sell our products profitably. Ongoing federal and state government initiatives directed at lowering the total cost of health care will likely continue to focus on healthcare reform, the cost of prescription pharmaceuticals and on the reform of the Medicare and Medicaid payment systems.
| 22 |
We expect that the pharmaceutical industry will continue to experience pricing pressures due to these initiatives and the trend toward managed healthcare and the increasing influence of managed care organizations. Our results of operations could be adversely affected by current and future healthcare reforms.
Some third party payors also require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers that use such therapies. While we cannot predict whether any proposed cost-containment measures will be adopted or otherwise implemented in the future, the announcement or adoption of these proposals could have a material adverse effect on our ability to obtain adequate prices for our EGP-437 Combination Product and operate profitably.
Other Regulatory Requirements
We are also subject to various laws and regulations regarding laboratory practices, the experimental use of animals, and the use and disposal of hazardous or potentially hazardous substances in connection with our research. In each of these areas, as above, the applicable regulatory agency will have broad regulatory and enforcement powers, including, among other things, the ability to levy fines and civil penalties. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental regulations may impair our research, development and production efforts, which could harm our business, operating results and financial condition.
Employees
As of December 31, 2017, we had seventeen full time employees.
Business Segment and Geographical Information
Operating segments are identified as components of an enterprise for which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision making group, in making decisions on how to allocate resources and assess performance. We view our operations and manage our business in one operating segment. We operate in one geographic segment (research and development).
Our Corporate Information
EyeGate Pharmaceuticals, Inc. was formed as a Delaware corporation on December 26, 2004. We were originally incorporated in 1998 under the name of Optis France S.A. in Paris, France. At the time of our incorporation in Delaware, the name of the French corporation was changed to EyeGate Pharma S.A.S. and became a subsidiary of EyeGate Pharmaceuticals, Inc. Our principal executive offices are located at 271 Waverley Oaks Road, Suite 108, Waltham, MA 02452, and our telephone number is (781) 788-9043.
| 23 |
Available Information and Website
We maintain an internet website at www.eyegatepharma.com and make available free of charge through our website our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Sections 13(a) and 15(d) of the Exchange Act. We make these reports available through our website as soon as reasonably practicable after we electronically file such reports with, or furnish such reports to, the United States Securities and Exchange Commission, or the SEC. You can find, copy and inspect information we file at the SEC’s public reference room, which is located at 100 F Street, N.E., Room 1580, Washington, DC 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC’s public reference room. You can review our electronically filed reports and other information that we file with the SEC on the SEC’s web site at http://www.sec.gov. We also make available, free of charge on our website, the reports filed with the SEC by our executive officers, directors and 10% stockholders pursuant to Section 16 under the Exchange Act as soon as reasonably practicable after copies of those filings are provided to us by those persons. The information on our website is not incorporated by reference into this Annual Report on Form 10-K and should not be considered to be a part of this Annual Report on Form 10-K. Our website address is included in this Annual Report on Form 10-K as an inactive technical reference only.
| 24 |
| Item 1A. | Risk Factors. |
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred significant operating losses since our inception. We expect to incur losses for the foreseeable future and may never achieve or maintain profitability.
Since inception, we have incurred significant operating losses. Our net loss was approximately $13.2 million for the year ended December 31, 2017, $13.3 million for the year ended December 31, 2016 and $91.8 million from the period of inception (December 26, 2004) through December 31, 2017. To date, we have financed our operations primarily through private placements of our preferred stock and convertible promissory notes, public offerings of our common stock, and payments from our license agreements. We have devoted substantially all of our financial resources and efforts to research and development, including preclinical studies and, beginning in 2008, clinical trials. We are still in the development stage of our product candidates and we have not completed development of any drugs. We expect to continue to incur significant expenses and operating losses for the foreseeable future. Our net losses may fluctuate significantly from quarter to quarter and year to year. Our recurring losses from operations have caused management to determine there is substantial doubt regarding our ability to continue as a going concern, and as a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2017 with respect to this uncertainty.
We anticipate that our expenses will continue to be significant with the clinical trials for our EGP-437 Combination Product, which consists of EGP-437 and our EyeGate® II Delivery System, as well as the ongoing development of our EyeGate OBG product.
Our expenses will also increase if and as we:
| · | pursue a safety clinical trial evaluating corneal endothelial cell counts over a six-month period with the EGP-437 Combination Product; |
| · | seek marketing approval for the EGP-437 Combination Product for anterior uveitis or post cataract surgery inflammation and pain in the U.S., as well as for EyeGate OBG, whether alone or in collaboration with third parties; |
| · | pursue the development of the EGP-437 Combination Product for the treatment of additional indications or for use in other patient populations or, if it is approved, seek to broaden the label for the EGP-437 Combination Product; |
| · | continue the research and development of our other product candidates, including EyeGate OBG; |
| · | seek to develop additional product candidates; |
| · | in-license or acquire the rights to other products, product candidates or technologies for the treatment of ophthalmic diseases; |
| 25 |
| · | seek marketing approvals for any product candidates that successfully complete clinical trials; |
| · | establish sales, marketing and distribution capabilities and scale up and validate external manufacturing capabilities to commercialize any products for which we may obtain marketing approval; |
| · | maintain, expand and protect our intellectual property portfolio; |
| · | hire additional clinical, quality control, scientific and management personnel; |
| · | expand our operational, financial and management systems and personnel, including personnel to support our clinical development, manufacturing and planned future commercialization efforts and our operations as a public company; and |
| · | increase our insurance coverage as we expand our clinical trials and commence commercialization of the EGP-437 Combination Product and EyeGate OBG. |
Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. Our expenses will increase if:
| · | we are required by the U.S. Food and Drug Administration, or FDA, or foreign equivalents, to perform studies or clinical trials in addition to those currently expected; |
| · | there are any delays in enrollment of patients in or completing our clinical trials or the development of the EGP-437 Combination Product, EyeGate OBG, or any other product candidates that we may develop. |
Our ability to become and remain profitable depends on our ability to generate revenue. We do not expect to generate significant revenue unless and until we obtain marketing approval for, and commercialize, the EGP-437 Combination Product, EyeGate OBG, or other product candidates that we may develop, which may never occur. This will require us to be successful in a range of challenging activities, including:
| · |
continuing and obtaining favorable results from a confirmatory Phase 3 clinical trial for the EGP-437 Combination Product for the treatment of non-infectious anterior uveitis and post cataract surgery inflammation and pain, and for the endothelial cell count safety trial;
|
|
| · | subject to obtaining favorable results from a confirmatory Phase 3 clinical trial for the EGP-437 Combination Product treating anterior uveitis and post cataract surgery inflammation and pain patients, applying for and obtaining marketing approval for the EGP-437 Combination Product; |
| 26 |
| · | establishing collaboration, distribution or other marketing arrangements with third parties to commercialize the EGP-437 Combination Product and EyeGate OBG in markets outside the U.S.; |
| · | achieving an adequate level of market acceptance of our product candidates; |
| · | protecting our rights to our intellectual property portfolio related to our product candidates; and |
| · | ensuring the manufacture of commercial quantities of the EGP-437 Combination Product and EyeGate OBG. |
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our product offerings or even continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will need substantial additional funding. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect to devote substantial financial resources to our ongoing and planned activities, particularly continuing our clinical trial evaluating the EGP-437 Combination Product for the treatments of uveitis and post-cataract surgery inflammation and pain, as well as developing our EyeGate OBG product. In the future, we expect to raise additional financial resources for the continued clinical development of the EGP-437 Combination Product, EyeGate OBG, and other product candidates we may develop. In addition, if we obtain regulatory approval for any of our product candidates, we would need to devote substantial financial resources to commercialization efforts, including product manufacturing, marketing, sales and distribution. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
Our future capital requirements will depend on many factors, including:
| · | the progress, costs and outcome of our clinical trials for our product candidates and of any clinical activities required for regulatory review of our product candidates outside of the U.S.; |
| · | the costs and timing of process development and manufacturing scale up and validation activities associated with our product candidates; |
| · | the costs, timing and outcome of regulatory review of our product candidates in the U.S., and in other jurisdictions; |
| · | the costs and timing of commercialization activities for our product candidates if we receive marketing approval, including the costs and timing of establishing product sales, marketing, distribution and outsourced manufacturing capabilities; |
| · | subject to receipt of marketing approval, the amount of revenue received from commercial sales of our product candidates; |
| 27 |
| · | the progress, costs and outcome of developing the EGP-437 Combination Product for the treatment of additional indications or for use in other patient populations; |
| · | our ability to establish collaborations on favorable terms, if at all, particularly manufacturing, marketing and distribution arrangements for our product candidates; |
| · | the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; and |
| · | the extent to which we in-license or acquire rights to other products, product candidates or technologies for the treatment of ophthalmic diseases. |
As of December 31, 2017, we had cash and cash equivalents of $7.8 million. We will have sufficient cash to fund planned operations for approximately seven months, however, the acceleration or reduction of cash outflows by management can significantly impact the timing for raising additional capital to complete development of its products. To continue development, we will need to raise additional capital through debt and/or equity financing, or access additional funding through grants. Although we completed our initial public offering, our August 2015 follow-on public offering, our June 2016 registered direct offering, sales under the ATM Agreement, and our June 2017 follow-on public offering, additional capital may not be available on terms favorable to us, if at all. Accordingly, no assurances can be given that management will be successful in these endeavors. These conditions raise substantial doubt about our ability to continue as a going concern. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities or any other adjustments that might be necessary should we be unable to continue as a going concern.
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. Our commercial revenues, if any, will be derived from sales of the EGP-437 Combination Product, EyeGate OBG or any other products that we successfully develop, none of which we expect to be commercially available for several years, if at all. In addition, if approved, the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we develop or any product that we in-license may not achieve commercial success. Accordingly, we will need to obtain substantial additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, government or other third-party funding, collaborations, strategic alliances, licensing arrangements and marketing and distribution arrangements. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we cannot raise funds on acceptable terms, we may not be able to grow our business or respond to competitive pressures.
| 28 |
If we raise additional funds through government or other third-party funding, collaborations, strategic alliances, licensing arrangements or marketing and distribution arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market products or product candidates that we would otherwise prefer to develop and market ourselves.
Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We are a clinical-stage company with a limited operating history. Our operations to date have been limited to organizing and staffing our company, acquiring rights to intellectual property, business planning, raising capital, developing our technology, identifying potential product candidates, undertaking preclinical studies and, beginning in 2008, conducting clinical trials of the EGP-437 Combination Product, as well as the EyeGate OBG. We have not yet demonstrated our ability to successfully complete development of a product candidate, obtain marketing approvals, manufacture at commercial scale, or arrange for a third party to do so on our behalf, or conduct sales, marketing and distribution activities necessary for successful product commercialization.
In addition, as a pre-revenue business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition at some point from a company with a research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We expect our financial condition and operating results to continue to fluctuate significantly from quarter-to-quarter and year-to-year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of future operating performance.
Risks Related to the Discovery and Development of Our Product Candidates
We depend heavily on the success of the EGP-437 Combination Product and EyeGate OBG. If we are unable to successfully obtain marketing approval for the EGP-437 Combination Product and EyeGate OBG, or experience significant delays in doing so, or if after obtaining marketing approvals, we fail to commercialize the EGP-437 Combination Product and EyeGate OBG, our business will be materially harmed.
We have invested a significant portion of our efforts and financial resources in the development of the EGP-437 Combination Product for the treatment of patients with non-infectious anterior uveitis and post cataract surgery inflammation and pain, and the EyeGate OBG. There remains a significant risk that we will fail to successfully develop the EGP-437 Combination Product and EyeGate OBG. In 2013, we completed a Phase 3 clinical trial to evaluate the safety, tolerability and efficacy of the EGP-437 Combination Product in patients with non-infectious anterior uveitis. In 2017, we also completed a Phase 2b clinical trial for post cataract surgery inflammation and pain. Our development plan for the EGP-437 Combination Product consists of a confirmatory Phase 3 clinical trial evaluating the EGP-437 Combination Product for the treatment of non-infectious anterior uveitis, which is currently in progress, a confirmatory Phase 3 clinical trial evaluating the EGP-437 Combination Product for post cataract surgery inflammation and pain, and a separate clinical trial evaluating corneal endothelial cell counts six months post treatment of the EGP-437 Combination Product. In 2017, we completed the first-in-human pilot trial of EyeGate OBG. Our development plan for EyeGate OBG consists of a second pilot trial that we anticipate commencing in the first half of 2018 and, assuming positive results from that trial, a subsequent pivotal trial we expect to initiate in the second half of 2018. We cannot accurately predict when or if the EGP-437 Combination Product and EyeGate OBG will prove effective or safe in humans or whether it will receive marketing approval. Our ability to generate product revenues, which may never occur, will depend heavily on our obtaining marketing approval for and commercializing the EGP-437 Combination Product and EyeGate OBG.
| 29 |
The success of the EGP-437 Combination Product and EyeGate OBG will depend on several factors, including the following:
| · | obtaining favorable results from confirmatory Phase 3 clinical trials for the EGP-437 Combination Product and for the endothelial cell count safety trial, and from the second trial and subsequent pivotal trial of EyeGate OBG; |
| · | applying for and receiving marketing approvals from applicable regulatory authorities for the EGP-437 Combination Product and EyeGate OBG; |
| · | making arrangements with third-party manufacturers for commercial quantities of EyeGate OBG and both the EGP-437 and the EyeGate® II Delivery System and receiving regulatory approval of our manufacturing processes and our third-party manufacturers’ facilities from applicable regulatory authorities; |
| · | establishing sales, marketing and distribution capabilities and launching commercial sales of the EGP-437 Combination Product and EyeGate OBG, if and when approved, whether alone or in collaboration with others; |
| · | acceptance of the EGP-437 Combination Product and EyeGate OBG, if and when approved, by patients, the medical community and third-party payors; |
| · | effectively competing with other therapies, including the existing standard of care; |
| · | maintaining a continued acceptable safety profile of the EGP-437 Combination Product and EyeGate OBG following approval; |
| · | obtaining and maintaining coverage and adequate reimbursement from third-party payors; |
| · | obtaining and maintaining patent and trade secret protection and regulatory exclusivity; and |
| · | protecting our rights in our intellectual property portfolio related to the EGP-437 Combination Product and EyeGate OBG. |
Successful development of the EGP-437 Combination Product for additional indications, if any, or for use in broader patient populations and our ability, if it is approved, to broaden the label for the EGP-437 Combination Product will depend on similar factors. If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize the EGP-437 Combination Product and/or EyeGate OBG, which would materially harm our business.
| 30 |
If clinical trials of the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we develop fail to demonstrate safety and efficacy to the satisfaction of the FDA or other regulatory authorities or do not otherwise produce favorable results, we may incur additional costs or experience delays in completing, or ultimately be delayed or unable to complete, the development and commercialization of the EGP-437 Combination Product, EyeGate OBG or any other product candidate.
Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, including our EGP-437 Combination Product or EyeGate OBG, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
We will be required to demonstrate the safety of the EGP-437 Combination Product by assessing corneal endothelial cell counts at six months from treatment in order to support marketing approval of the EGP-437 Combination Product for the treatment of non-infectious anterior uveitis in the U.S. To meet this requirement in the future after raising additional funds, we plan to conduct a separate safety trial with no fewer than 100 patients who will be treated with the EGP-437 Combination Product and followed for six months post treatment. We cannot predict the results of this safety trial because we have no clinical data supporting the effect of our EGP-437 Combination Product on corneal endothelial cells six months post treatment.
In general, the FDA requires two adequate and well controlled pivotal clinical trials demonstrating effectiveness on a primary endpoint for marketing approval of a non-infectious anterior uveitis drug. The endpoint is based on total clearance of inflammatory cells in the anterior chamber of the eye. The trial must compare the EGP-437 Combination Product to standard of care. Our first Phase 3 trial evaluated the EGP-437 Combination Product for the treatment of non-infectious anterior uveitis against a positive control, the standard of care, prednisolone acetate ophthalmic suspension (1%), or PA. In our Phase 3 trial, the dose of the EGP-437 Combination Product tested was just outside the pre-set non-inferiority margin for intent-to-treat and per protocol populations and did not achieve statistical significance in the intent-to-treat population as compared to the positive control based on the primary efficacy endpoint.
We may fail to achieve success in our confirmatory Phase 3 clinical trials evaluating the EGP-437 Combination Product for the treatment of non-infectious anterior uveitis or post cataract surgery inflammation and pain for a variety of potential reasons. Even if our confirmatory Phase 3 trials are successful in showing confirmatory data, the FDA may still require us to provide additional data to grant regulatory approval.
We are conducting our confirmatory Phase 3 clinical trial for the treatment of non-infectious anterior uveitis at many clinical centers that were not included in our first Phase 3 trial. The introduction of new centers, and the resulting involvement of new treating physicians, can introduce additional variability into the conduct of the trials in accordance with their protocols and may result in greater variability of patient outcomes, which could adversely affect our ability to detect statistically significant differences between patients treated with the EGP-437 Combination Product and the standard of care control.
If, in our confirmatory Phase 3 clinical trials, we do not demonstrate non-inferiority as compared with the standard of care and if the FDA does not find this to be an acceptable means of meeting the requirements for marketing approval, we will not receive marketing approval for the EGP-437 Combination Product, and we will have to conduct another Phase 3 clinical trial if we wish to seek marketing approval for the EGP-437 Combination Product in the future.
The protocol for our confirmatory Phase 3 clinical trials and other supporting information are subject to review by the FDA and regulatory authorities outside the U.S. We did not submit the protocols for our second confirmatory Phase 3 clinical trials and do not plan on submitting the protocols for our separate safety trial of the EGP-437 Combination Product to the FDA at any time prior to the raising of additional funds. We have not received guidance from other regulatory authorities outside the U.S. regarding the design of our confirmatory Phase 3 clinical trials.
| 31 |
Our confirmatory Phase 3 clinical trial for the treatment of non-infectious anterior uveitis has a non-inferiority design. We may be unable to demonstrate non-inferiority against the standard of care, PA, which may cause us to undergo additional clinical trials or admit additional subjects to our trials delaying the time and increasing the expense it may take to commercialize our EGP-437 Combination Product.
Our confirmatory Phase 3 clinical trial for the treatment of non-infectious anterior uveitis uses a non-inferiority design rather than a superiority design. In order to meet our primary endpoint, we must show that patients treated with the EGP-437 Combination Product demonstrate non-inferiority according to pre-set non-inferiority margins as compared with the standard of care, PA. We may be unable to demonstrate non-inferiority against the standard of care. The design and conduct of non-inferiority trials, including selection of non-inferiority margins, account for many factors that can induce bias in the estimated effect of the standard of care in the non-inferiority trial and thus lead to bias in the estimated effect of the experimental treatment and perhaps lead to a trial design that does not ensure that the experimental treatment preserves a clinically acceptable fraction of the standard’s effect, which may result in a vulnerability of the integrity of a non-inferiority trial to the irregularities in trial conduct. Our choice of an endpoint based on total clearance of inflammatory cells in the anterior chamber of the eye means that success will depend to a significant degree on the accuracy of our assumptions about the total clearance of inflammatory cells in the anterior chamber of the eye in the comparator arms of our Phase 3 trial. Although we believe we have been conservative in our assumptions, if, for example, patients in the comparator arm of our trial have significantly different clearance of inflammatory cells than we expect, we may find that our trial is unfeasible or we may have to enroll more patients at additional cost and delay.
If we experience any of a number of possible unforeseen events in connection with our clinical trials, potential marketing approval or commercialization of our product candidates could be delayed or prevented.
We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive marketing approval or commercialize the EGP-437 Combination Product, EyeGate OBG or any other product candidates that we may develop, including:
| · | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; |
| · | the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate; |
| · | any third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| · | regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| · | we may experience delays in reaching, or fail to reach, agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
| · | we may decide, or regulators or institutional review boards may require us, to suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; |
| 32 |
| · | the cost of clinical trials of our product candidates may be greater than we anticipate; |
| · | the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate; and |
| · | our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or institutional review boards to suspend or terminate the trials. |
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are not favorable or are only modestly favorable or if there are safety concerns, we may:
| · | be delayed in obtaining marketing approval for our product candidates; |
| · | not obtain marketing approval at all; |
| · | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| · | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| · | be subject to additional post-marketing testing requirements; or |
| · | have the product removed from the market after obtaining marketing approval. |
Our product development costs will also increase if we experience delays in testing or marketing approvals. We do not know whether any of our pre-clinical studies or clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Significant preclinical or clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our ability to successfully commercialize our product candidates.
If we experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for the EGP-437 Combination Product, EyeGate OBG or our other product candidates that we may develop if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the U.S. In addition, some of our competitors may have ongoing clinical trials for product candidates that treat the same indications as the EGP-437 Combination Product, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates.
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays, could require us to abandon one or more clinical trials altogether and could delay or prevent our receipt of necessary regulatory approvals. Enrollment delays in our clinical trials may result in increased development costs for our product candidates, which would cause the value of our company to decline and limit our ability to obtain additional financing.
| 33 |
If serious adverse or unacceptable side effects are identified during the development of our product candidates, we may need to abandon or limit our development of such product candidates.
If the EGP-437 Combination Product, EyeGate OBG or any of our other product candidates are associated with serious adverse events or undesirable side effects in clinical trials or have characteristics that are unexpected, we may need to abandon their development or limit development to more narrow uses or subpopulations in which the serious adverse events, undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Many compounds that initially showed promise in clinical or early stage testing for treating ophthalmic disease have later been found to cause side effects that prevented further development of the compound.
We may not be successful in our efforts to use our EyeGate® II Delivery System or platform to build a pipeline of product candidates.
A key element of our strategy is to use our proprietary EyeGate® II Delivery System or platform to rationally design, engineer and generate a pipeline of products and progress these therapies through clinical development for the treatment of a variety of ophthalmic diseases. Our research and development efforts to date have resulted in a pipeline of additional product candidates directed at the treatment of ophthalmic diseases. These and any other potential product candidates that we identify may not be suitable for continued preclinical or clinical development, including as a result of being shown to have harmful side effects or other characteristics that indicate that they are unlikely to be products that will receive marketing approval and achieve market acceptance. If we do not successfully develop and commercialize our product candidates that we develop based upon our technological approach, we will not be able to obtain product revenues in future periods.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. To the extent our contemplated trials are unsuccessful, we may not be able to raise additional funds for subsequent trials or pursuing other indications.
| 34 |
Risks Related to the Commercialization of Our Product Candidates
Even if the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we develop receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success and the market opportunity for the EGP-437 Combination Product and EyeGate OBG may be smaller than we estimate.
If the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we develop receives marketing approval, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community.
Current treatments that are used for anterior uveitis and inflammation post cataract surgery include topical corticosteroids such as Durezol® (Novartis AG), Lotemax® (Valeant Pharmaceuticals International, Inc.), Pred Forte® (Allergan, Inc.) and prednisolone acetate ophthalmic suspension (1%) (Novartis AG), as well as topical NSAIDs such as Bromfenac. These treatments are well established in the medical community, and doctors may continue to rely on these treatments rather than our EGP-437 Combination Product, if and when it is approved for marketing by the FDA.
Current treatment or standard-of-care post photorefractive keratectomy (PRK) surgery is a bandage contact lens (BCL), such as the Acuvue Oasys® (Johnson & Johnson Vision Care, Inc.), along with a variety of topical antibiotics and anti-inflammatories, which are available from several suppliers. BCL as treatments post PRK surgery are well established in the medical community, and doctors may continue to rely on these treatments rather than the EyeGate OBG Product, if and when it is cleared for marketing by the FDA.
Our assessment of the potential market opportunity for the EGP-437 Combination Product and EyeGate OBG is based on industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. If the actual market for the EGP-437 Combination Product and EyeGate OBG is smaller than we expect, our product revenue may be limited and it may be more difficult for us to achieve or maintain profitability.
If we are unable to establish sales, marketing and distribution capabilities, we may not be successful in commercializing the EGP-437 Combination Product, EyeGate OBG or any other product candidates that we may develop if and when they are approved.
We do not have a sales or marketing infrastructure. To achieve commercial success for any product for which we have obtained marketing approval and have not licensed the commercialization rights, we will need to establish sales, marketing and distribution capabilities, either ourselves or through collaborations or other arrangements with third parties as we have under the Valeant license agreements.
In the future, we plan to build an ophthalmic focused sales and marketing infrastructure to market or co-promote the EyeGate OBG product and possibly other product candidates that we develop in the U.S., if and when they are approved. There are risks involved with establishing our own sales, marketing and distribution capabilities. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of the EyeGate OBG or any other product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize the EGP-437 Combination Product, EyeGate OBG or any other product candidates on our own include:
| · | our inability to recruit, train and retain adequate numbers of effective sales and marketing personnel; |
| · | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe our products; |
| 35 |
| · | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| · | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
We expect to enter into arrangements with third parties to perform consulting, sales, marketing and distribution services in markets outside the U.S. We may also enter into arrangements with third parties to perform these services in the U.S. if we do not establish our own sales, marketing and distribution capabilities in the U.S. or if we determine that such third-party arrangements are otherwise beneficial. Our product revenues and our profitability, if any, under any such third-party sales, marketing or distribution arrangements are likely to be lower than if we were to market, sell and distribute the EGP-437 Combination Product, EyeGate OBG or any other product candidates that we may develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute the EyeGate OBG product or any other product candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market the EGP-437 Combination Product, EyeGate OBG or our other product candidates effectively. If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our EGP-437 Combination Product, EyeGate OBG or any other product candidates that we may develop.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to the EGP-437 Combination Product, EyeGate OBG and our other current product candidates, and will face competition with respect to any product candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our product candidates. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
The current standard of care for non-infectious anterior uveitis and inflammation post cataract surgery include topical corticosteroids such as Durezol® (Novartis AG), Lotemax® (Valeant Pharmaceuticals International, Inc.), Pred Forte® (Allergan, Inc.) and prednisolone acetate ophthalmic suspension (1%) (Novartis AG), as well as topical NSAIDs such as Bromfenac. Current treatment or standard-of-care post PRK surgery is a BCL, such as the Acuvue Oasys® (Johnson & Johnson Vision Care, Inc.) along with a variety of topical antibiotics and anti-inflammatories, which are available from several suppliers.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than the EGP-437 Combination Product, EyeGate OBG or other product candidates that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
In addition, our ability to compete may be affected in many cases by insurers or other third-party payors, particularly Medicare, seeking to encourage the use of generic products. Generic products are currently being used for the indications that we are pursuing, and additional products are expected to become available on a generic basis over the coming years. If the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we may develop achieves marketing approval, we expect that it will be priced at a premium over competitive products.
| 36 |
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if we are able to commercialize the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we may develop, the products may become subject to unfavorable pricing regulations, third-party coverage or reimbursement practices or healthcare reform initiatives, which could harm our business.
Our ability to commercialize the EGP-437 Combination Product, EyeGate OBG or any other product candidates that we may develop successfully will depend, in part, on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government healthcare programs, private health insurers, managed care plans and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Coverage and reimbursement may not be available for the EGP-437 Combination Product, EyeGate OBG or any other product that we commercialize and, even if they are available, the level of reimbursement may not be satisfactory.
Inadequate reimbursement may adversely affect the demand for, or the price of, any product candidate for which we obtain marketing approval. Obtaining and maintaining adequate reimbursement for our products may be difficult. We may be required to conduct expensive pharmacoeconomic studies to justify coverage and reimbursement or the level of reimbursement relative to other therapies. If coverage and adequate reimbursement are not available or reimbursement is available only to limited levels, we may not be able to successfully commercialize the EGP-437 Combination Product, EyeGate OBG or any other product candidate for which we obtain marketing approval.
There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the indications for which the drug is approved by the FDA or similar regulatory authorities outside the U.S. Moreover, eligibility for coverage and reimbursement does not imply that a drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution expenses. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and adequate reimbursement rates from both government-funded and private payors for any approved products that we develop would compromise our ability to generate revenues and become profitable.
| 37 |
The regulations that govern marketing approvals, pricing, coverage and reimbursement for new drug products vary widely from country to country. Current and future legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing approval.
There can be no assurance that our product candidates or any products that we may in-license, if they are approved for sale in the U.S. or in other countries, will be considered medically reasonable and necessary for a specific indication, that they will be considered cost-effective by third-party payors, that coverage and an adequate level of reimbursement will be available, or that third-party payors’ reimbursement policies will not adversely affect our ability to sell our product candidates profitably.
Our strategy of obtaining rights to product candidates and approved products for the treatment of a range of ophthalmic diseases through in-licenses and acquisitions may not be successful.
We may expand our product pipeline through opportunistically in-licensing or acquiring the rights to other products, product candidates or technologies for the treatment of ophthalmic diseases. The future growth of our business may depend in part on our ability to in-license or acquire the rights to approved products, additional product candidates or technologies. However, we may be unable to in-license or acquire the rights to any such products, product candidates or technologies from third parties. The in-licensing and acquisition of pharmaceutical products is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire products, product candidates or technologies that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to in-license or acquire the rights to the relevant product, product candidate or technology on terms that would allow us to make an appropriate return on our investment. Furthermore, we may be unable to identify suitable products, product candidates or technologies within our area of focus. If we are unable to successfully obtain rights to suitable products, product candidates or technologies, our ability to pursue this element of our strategy could be impaired.
| 38 |
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we develop.
We face an inherent risk of product liability exposure related to the use of the EGP-437 Combination Product, EyeGate OBG, and any other product candidates that we develop in human clinical trials and will face an even greater risk if we commercially sell any products that we develop. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| · | decreased demand for any product candidates or products that we develop; |
| · | injury to our reputation and significant negative media attention; |
| · | withdrawal of clinical trial participants; |
| · | significant costs to defend the related litigation; |
| · | substantial monetary awards to trial participants or patients; |
| · | loss of revenue; |
| · | reduced time and attention of our management to pursue our business strategy; and |
| · | the inability to commercialize any products that we develop. |
While we obtain insurance for each clinical trial we perform, we may not be adequately insured to cover all liabilities that we may incur. We will need to increase our insurance coverage as we expand our clinical trials. We will need to further increase our insurance coverage if we commence commercialization of the EGP-437 Combination Product, EyeGate OBG or any other product candidate that receives marketing approval. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Risks Related to Our Dependence on Third Parties
We may enter into collaborations with other third parties for the development or commercialization of our product candidates, including the EGP-437 Combination Product and EyeGate OBG. If our collaborations are not successful, we may not be able to capitalize on the market potential of these product candidates.
We expect to utilize a variety of types of collaboration, distribution and other marketing arrangements with third parties to commercialize the EGP-437 Combination Product and EyeGate OBG in markets outside the U.S. We also may enter into arrangements with third parties to perform these services in the U.S. if we do not establish our own sales, marketing and distribution capabilities in the U.S. or if we determine that such third-party arrangements are otherwise beneficial. We also may seek third-party collaborators for development and commercialization of other product candidates. Our likely collaborators for any sales, marketing, distribution, development, licensing or broader collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. To date, the only agreements we have entered into are our Valeant Licensing Agreements. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities and efforts to successfully perform the functions assigned to them in these arrangements.
| 39 |
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner, or at all. If we do not receive the funding we expect under collaboration agreements, our development of our product candidates could be delayed and we may need additional resources to develop our product candidates. All of the risks relating to product development, regulatory approval and commercialization described in this Annual Report on Form 10-K also apply to the activities of our collaborators.
Additionally, subject to its contractual obligations to us, if a collaborator of ours were to be involved in a business combination, it might deemphasize or terminate the development or commercialization of any product candidate licensed to it by us. If one of our collaborators terminates its agreement with us, we may find it more difficult to attract new collaborators and our perception in the business and financial communities could be harmed.
If we are not able to establish additional collaborations, we may have to alter our development and commercialization plans and our business could be adversely affected.
For some of our product candidates, we may decide to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of therapeutic products. We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the U.S., the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. We may also be restricted under future license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
If we are unable to reach agreements with suitable collaborators on a timely basis, on acceptable terms, or at all, we may have to curtail the development of a product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to fund and undertake development or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we fail to enter into collaborations and do not have sufficient funds or expertise to undertake the necessary development and commercialization activities, we may not be able to further develop our product candidates or bring them to market or continue to develop our product platform.
Disputes may arise under our Valeant License Agreements, including disputes related to the scope of rights granted thereunder.
Disputes may arise under our Valeant License Agreements, including disputes related to the scope of rights granted thereunder. Any such disputes could lead to delays in the development or commercialization of our EGP-437 Combination Product and could result in time-consuming and expensive litigation or arbitration, which may not be resolved in our favor. Either party may terminate the Valeant License Agreements in their entirety if the other party materially breaches either Valeant License Agreement and the breach remains uncured for a defined cure period, and either party may terminate either Valeant License Agreement in its entirety upon the bankruptcy of the other party. We may terminate either Valeant License Agreement following commercial launch of our EGP 437-Combination Product if Valeant ceases selling and distributing our EGP 437-Combination Product in the United States for a defined period of time, subject to certain limitations. Valeant may terminate either Valeant License Agreement at any time, on a without cause basis, by providing 90 days written notice, or immediately upon the determination by a court of competent jurisdiction if Valeant’s actions pursuant to the terms of the Valeant License Agreement infringe upon the intellectual property rights of a third party. We cannot make assurances that these agreements will not be terminated in accordance with these terms, and such termination could have a material adverse impact on our future business, results of operations, financial conditions, and the trading price of our common stock.
| 40 |
We rely, and expect to continue to rely, on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.
We have relied on third parties, such as contract research organizations, or CROs, to conduct our completed trials of our EGP-437 Combination Product, our ongoing confirmatory Phase 3 clinical trial of our EGP-437 Combination Product and do not plan to independently conduct clinical trials of the EGP-437 Combination Product or other product candidates that we may develop, including EyeGate OBG. We expect to continue to rely on third parties, such as CROs, clinical data management organizations, medical institutions and clinical investigators, to conduct our clinical trials. These agreements might terminate for a variety of reasons, including a failure to perform by the third parties. If we need to enter into alternative arrangements, that would delay our product development activities.
Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within specified timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We contract with third parties for the manufacture of the EGP-437 Combination Product and EyeGate OBG for clinical trials and expect to continue to do so in connection with the commercialization of the EGP-437 Combination Product, EyeGate OBG, and for clinical trials and commercialization of any other product candidates that we may develop. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We do not currently own or operate manufacturing facilities for the production of clinical or commercial quantities of the EGP-437 Combination Product, EyeGate OBG, or any other of our product candidates. We rely, and expect to continue to rely, on third parties to manufacture clinical and commercial supplies of the EGP-437 Combination Product, EyeGate OBG, preclinical and clinical supplies of our other product candidates that we may develop, and commercial supplies of products if and when any of our product candidates receives marketing approval. Our current and anticipated future dependence upon others for the manufacture of the EGP-437 Combination Product, EyeGate OBG and any other product candidate or product that we develop may adversely affect our future profit margins and our ability to commercialize any products that receive marketing approval on a timely and competitive basis. In addition, any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval.
We currently rely exclusively on third-party manufacturers to assemble and prepare the EGP-437 Combination Product and EyeGate OBG on a purchase order basis. We do not currently have any contractual commitments for commercial supply of bulk drug substance for EGP-437, EyeGate OBG, or fill-finish services or for components of the EyeGate® II Delivery System. We also do not currently have arrangements in place for redundant supply or a second source for bulk drug substance for EGP-437, EyeGate OBG, or for fill-finish services. The prices at which we are able to obtain supplies of EGP-437, EyeGate OBG, fill-finish services, and assemble the EyeGate® II Delivery System may vary substantially over time and adversely affect our financial results.
| 41 |
If our third-party manufacturers for the EGP-437 Combination Product or EyeGate OBG fail to fulfill our purchase orders or should become unavailable to us for any reason, we believe that there are a limited number of potential replacement manufacturers, and we likely would incur added costs and delays in identifying or qualifying such replacements. We could also incur additional costs and delays in identifying or qualifying a replacement manufacturer for fill-finish services if our existing third-party manufacturer should become unavailable for any reason. We may be unable to establish any agreements with such replacement manufacturers or to do so on acceptable terms. Even if we could transfer manufacturing to a different third party, the shift would likely be expensive and time consuming, particularly since the new facility would need to comply with the necessary regulatory requirements and we would need FDA approval before using or selling any products manufactured at that facility.
In connection with our application for a license to market the EGP-437 Combination Product, EyeGate OBG, or other product candidates in the U.S., we may be required to conduct a comparability study if the product we intend to market is supplied by a manufacturer different from the one who supplied the product evaluated in our clinical studies. Delays in designing and completing this study to the satisfaction of the FDA could delay or preclude our development and commercialization plans and thereby limit our revenues and growth.
Reliance on third-party manufacturers entails additional risks, including:
| · | the EGP-437 Combination Product, EyeGate OBG and any other product candidates that we may develop may compete with other product candidates and products for access to a limited number of suitable manufacturing facilities that operate under current good manufacturing practices, or cGMP, regulations; |
| · | reliance on the third party for regulatory compliance and quality assurance; |
| · | the possible breach of the manufacturing agreement by the third party; |
| · | the possible misappropriation of our proprietary information, including our trade secrets and know-how; and |
| · | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. |
Third-party manufacturers may not be able to comply with cGMP regulations or similar regulatory requirements outside the U.S. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products.
| 42 |
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain patent protection for our technology and products or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be impaired.
Our success depends in large part on our ability to obtain and maintain patent protection in the U.S. and other countries with respect to our proprietary technology and products. We and our licensors have sought to protect our proprietary position by filing patent applications in the U.S. and abroad related to our novel technologies and product candidates. The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Maintaining patents in the U.S. is an expensive process and it is even more expensive to maintain patents and patent applications in foreign countries. As a result, it is possible that we and our licensors will fail to maintain such patents thereby reducing the rights of our portfolio.
The patent position of pharmaceutical, biotechnology and medical device companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our and our licensors’ patent rights are highly uncertain. Our and our licensors’ pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the U.S. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our owned or licensed patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. In particular, during prosecution of any patent application, the issuance of any patents based on the application may depend upon our ability to generate additional preclinical or clinical data that support the patentability of our proposed claims. We may not be able to generate sufficient additional data on a timely basis, or at all. Moreover, changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may diminish the value of our patents or narrow the scope of our patent protection.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The U.S. Patent Office recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
| 43 |
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the U.S. Patent and Trademark Office, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our issued patents or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents. In addition, in a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability, and the ability of our collaborators, to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the medical device, biotechnology and pharmaceutical industries. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference or derivation proceedings before the U.S. Patent and Trademark Office. The risks of being involved in such litigation and proceedings may increase as our product candidates near commercialization and as we gain the greater visibility associated with being a public company. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. We may not be aware of all such intellectual property rights potentially relating to our product candidates and their uses. Thus, we do not know with certainty that the EGP-437 Combination Product, EyeGate OBG, or any other product candidate, or our commercialization thereof, does not and will not infringe or otherwise violate any third party’s intellectual property.
| 44 |
If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
If we fail to comply with our obligations in our intellectual property licenses and funding arrangements with third parties, we could lose rights that are important to our business.
We are party to licensing agreements, including the Valeant Licensing Agreements, that impose, and, for a variety of purposes, we will likely enter into additional licensing and funding arrangements with third parties that may impose, diligence, development and commercialization timelines and milestone payment, royalty, insurance and other obligations on us. Under certain of our existing licensing agreements, we are obligated to pay royalties or make specified milestone payments on net product sales of the EyeGate® II Delivery System or related technologies to the extent they are covered by the agreements. We also are obligated under certain of our existing license agreements to pay maintenance and other fees. We also have diligence and development obligations under certain of those agreements that we are required to satisfy. If we fail to comply with our obligations under current or future license and collaboration agreements, our counterparties may have the right to terminate these agreements, in which event we might not be able to develop, manufacture or market any product that is covered by these agreements or may face other penalties under the agreements. Such an occurrence could diminish the value of the product candidate being developed under any such agreement. Termination of these agreements or reduction or elimination of our rights under these agreements may result in our having to negotiate new or reinstated agreements with less favorable terms, or cause us to lose our rights under these agreements, including our rights to important intellectual property or technology.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Some of our employees were previously employed at universities or other biotechnology or pharmaceutical companies. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims.
In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
| 45 |
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the U.S. are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
| 46 |
Risks Related to Regulatory Approval of Our Product Candidates and Other Legal Compliance Matters
If we are not able to obtain required regulatory approvals, we will not be able to commercialize the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we may develop, and our ability to generate revenue will be materially impaired. The marketing approval process is expensive, time-consuming and uncertain. As a result, we cannot predict when or if we, or any collaborators we may have in the future, will obtain marketing approval to commercialize the EGP-437 Combination Product, EyeGate OBG or any other product candidate.
The activities associated with the development and commercialization of our product candidates, including the EGP-437 Combination Product and EyeGate OBG, including design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the U.S. and similar regulatory authorities outside the U.S. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have not received approval to market the EGP-437 Combination Product, EyeGate OBG or any other product candidate from regulatory authorities in any jurisdiction. We have only limited experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-party CROs and consultants to assist us in this process.
The process of obtaining marketing approvals, both in the U.S. and abroad, is expensive and may take many years, especially if additional clinical trials are required, if approval is obtained at all. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety, purity and potency. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. The FDA or other regulatory authorities may determine that the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we may develop is not safe, effective or pure, is only moderately effective or has undesirable or unintended side effects, toxicities or other characteristics that preclude our obtaining marketing approval or prevent or limit commercial use. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
The regulatory process can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved.
Failure to obtain marketing approval in international jurisdictions would prevent our product candidates from being marketed abroad.
In order to market and sell the EGP-437 Combination Product, EyeGate OBG and any other product candidate that we may develop in other jurisdictions, we or our third-party collaborators must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the U.S. generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the U.S., it is required that the product be approved for reimbursement before the product can be sold in that country. We or these third parties may not obtain approvals from regulatory authorities outside the U.S. on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the U.S. does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market.
| 47 |
Even if we, or any collaborators we may have in the future, obtain marketing approvals for the EGP-437 Combination Product, EyeGate OBG or our other product candidates, the terms of those approvals, ongoing regulations and post-marketing restrictions may limit how we, or they, manufacture and market our products, which could materially impair our ability to generate revenue.
Once marketing approval has been granted, an approved product and its manufacturer and marketer are subject to ongoing review and extensive regulation. We, and any collaborators we may have in the future, must therefore comply with requirements concerning advertising and promotion for any of our products for which we or they obtain marketing approval. Promotional communications with respect to prescription products are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved labeling. Thus, if the EGP-437 Combination Product, EyeGate OBG or any other product candidate that we may develop receives marketing approval, the accompanying label may limit the approved use of our product, which could limit sales of the product.
In addition, manufacturers of approved products and those manufacturers’ facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to cGMPs, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We, our contract manufacturers, our future collaborators and their contract manufacturers will also be subject to other regulatory requirements, including submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements regarding the distribution of samples to physicians, recordkeeping, and costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product such as the requirement to implement a risk evaluation and mitigation strategy.
We may be subject to substantial penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products.
Violations of the Federal Food, Drug, and Cosmetic Act relating to the promotion or manufacturing of prescription products may lead to investigations by the FDA, Department of Justice and state Attorneys General alleging violations of federal and state healthcare fraud and abuse laws, as well as state consumer protection laws. In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various adverse results, including:
| · | restrictions on such products, manufacturers or manufacturing processes; |
| · | restrictions on the labeling or marketing of a product; |
| · | restrictions on product distribution or use; |
| · | requirements to conduct post-marketing studies or clinical trials; |
| · | warning letters; |
| · | withdrawal of the products from the market; |
| · | refusal to approve pending applications or supplements to approved applications that we submit; |
| · | recall of products; |
| 48 |
| · | fines, restitution or disgorgement of profits or revenues; |
| · | suspension or withdrawal of marketing approvals; |
| · | refusal to permit the import or export of our products; |
| · | product seizure; or |
| · | injunctions or the imposition of civil or criminal penalties. |
Our relationships with customers and third-party payors may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security, and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors in the U.S. and elsewhere will play a primary role in the recommendation and prescription of any product candidates, including the EGP-437 Combination Product and EyeGate OBG, for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any products for which we obtain marketing approval. In addition, we may be subject to transparency laws and patient privacy regulation by U.S. federal and state governments and by governments in foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include:
| · | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid; |
| · | federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, which impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| · | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and their respective implementing regulations, which imposes obligations, including mandatory contractual terms, on covered healthcare providers, health plans and healthcare clearinghouses, as well as their business associates, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; and |
| 49 |
| · | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, it may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government funded healthcare programs.
Recently enacted and future legislation may affect our ability to commercialize and the prices we obtain for any products that are approved in the U.S. or foreign jurisdictions.
In the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could affect our ability to profitably sell or commercialize any product candidate, including the EGP-437 Combination Product and EyeGate OBG, for which we obtain marketing approval or that we may in-license. The pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by legislative initiatives. Current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product.
In the U.S., the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, changed the way Medicare covers and pays for pharmaceutical products. Cost reduction initiatives and other provisions of this legislation could limit coverage of and reduce the price that we receive for any approved products. While the MMA applies only to product benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the MMA or other healthcare reform measures may result in a similar reduction in payments from private payors.
In March 2010, former President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively PPACA. Among the provisions of PPACA of importance to our business, including, without limitation, our ability to commercialize and the prices we may obtain for any of our product candidates and that are approved for sale, are the following:
| · | an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents; |
| 50 |
| · | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
| · | a new Medicare Part D coverage gap discount program, in which participating manufacturers must agree to offer 50% point-of-sale discounts off negotiated drug prices during the coverage gap period as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; |
| · | expansion of healthcare fraud and abuse laws, including the federal False Claims Act and the federal Anti-Kickback Statute, and the addition of new government investigative powers, and enhanced penalties for noncompliance; |
| · | extension of manufacturers’ Medicaid rebate liability; |
| · | expansion of eligibility criteria for Medicaid programs; and |
| · | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program. |
In addition, other legislative changes have been proposed and adopted since PPACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013. In January 2013, former President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several types of providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding. In addition, it is possible that changes in administration and policy, including the potential repeal of all or parts of the PPACA, resulting from the recent U.S. presidential election could result in additional proposals and/or changes to health care system legislation.
The pricing of prescription pharmaceuticals is also subject to governmental control outside the U.S. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our ability to generate revenues and become profitable could be impaired.
Laws and regulations governing any international operations we may have in the future may preclude us from developing, manufacturing and selling certain products outside of the U.S. and require us to develop and implement costly compliance programs.
If we expand our operations outside of the U.S., we must dedicate additional resources to comply with numerous laws and regulations in each jurisdiction in which we plan to operate. The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering, authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the U.S. to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
| 51 |
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. Certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
Various laws, regulations and executive orders also restrict the use and dissemination outside of the U.S., or the sharing with certain non-U.S. nationals, of information classified for national security purposes, as well as certain products and technical data relating to those products. If we expand our presence outside of the U.S., it will require us to dedicate additional resources to comply with these laws, and these laws may preclude us from developing, manufacturing, or selling certain products and product candidates outside of the U.S., which could limit our growth potential and increase our development costs.
The failure to comply with laws governing international business practices may result in substantial civil and criminal penalties and suspension or debarment from government contracting. The SEC also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA’s accounting provisions.
If we or our third-party manufacturers fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur significant costs.
We and our third-party manufacturers are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. From time to time and in the future, our operations may involve the use of hazardous and flammable materials, including chemicals and biological materials, and produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees, this insurance may not provide adequate coverage against potential liabilities.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Further, with respect to the operations of our third-party contract manufacturers, it is possible that if they fail to operate in compliance with applicable environmental, health and safety laws and regulations or properly dispose of wastes associated with our products, we could be held liable for any resulting damages, suffer reputational harm or experience a disruption in the manufacture and supply of our product candidates or products.
| 52 |
Risks Related to Employee Matters and Managing Growth
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on the research and development, clinical and business development expertise of Stephen From, our President and Chief Executive Officer, as well as the other principal members of our management, scientific and clinical team and a number of third party consultants. Although we have entered into an employment agreement with Mr. From, he may terminate his employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of and commercialize products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems, and those of our CROs and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
We expect to expand our development capabilities and potentially implement sales, marketing and distribution capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs and, if any of our product candidates receives marketing approval, sales, marketing and distribution. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We may fail to realize any benefits and incur losses related to any acquisition.
The success of our strategic acquisitions will depend, in part, on our ability to successfully integrate the acquired businesses with our existing business. It is possible that the integration process could result in the loss of key employees, the disruption of ongoing business or inconsistencies in standards, controls, procedures and policies that adversely affect our ability to maintain relationships with clients, customers and employees or to achieve the anticipated benefits of the acquisition. Successful integration may also be hampered by any differences between the operations and corporate culture of the two organizations. If we experience difficulties with the integration process, the anticipated benefits of the acquisition may not be realized fully, or at all, or may take longer to realize than expected.
| 53 |
Risks Related to Our Common Stock
Our principal stockholders, executive officers and directors own a significant percentage of our common stock and will be able to exert a significant control over matters submitted to the stockholders for approval.
Our officers and directors, and stockholders who own more than 5% of our common stock, beneficially own a significant percentage of our common stock. This significant concentration of share ownership may adversely affect the trading price for our common stock because investors often perceive disadvantages in owning stock in companies with concentrated ownership. These stockholders, if they acted together, could significantly influence all matters requiring approval by the stockholders, including the election of directors. The interests of these stockholders may not always coincide with the interests of other stockholders.
Provisions in our corporate charter documents and under Delaware law could make an acquisition of our company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our restated certificate of incorporation and our amended and restated bylaws may discourage, delay or prevent a merger, acquisition or other change in control of our company that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
| · | establish a classified board of directors such that only one of three classes of directors is elected each year; |
| · | allow the authorized number of our directors to be changed only by resolution of our board of directors; |
| · | limit the manner in which stockholders can remove directors from our board of directors; |
| · | establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors; |
| · | require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent; |
| · | limit who may call stockholder meetings; |
| · | authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and |
| · | require the affirmative vote of stockholders holding at least two-thirds of shares entitled to be cast to amend or repeal specified provisions of our restated certificate of incorporation or our amended and restated bylaws. |
| 54 |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for purchasers of our common stock.
Our stock price may be volatile. The stock market in general and the market for smaller specialty pharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your common stock at or above the price you paid for such shares. The market price for our common stock may be influenced by many factors, including:
| · | the success of competitive products or technologies; |
| · | results of clinical trials of the EGP-437 Combination Product or any other product candidate that we may develop; |
| · | results of clinical trials of product candidates of our competitors; |
| · | regulatory or legal developments in the U.S. and other countries; |
| · | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| · | the recruitment or departure of key scientific or management personnel; |
| · | the level of expenses related to any of our product candidates or clinical development programs; |
| · | the results of our efforts to discover, develop, acquire or in-license additional products, product candidates or technologies for the treatment of ophthalmic diseases, the costs of commercializing any such products and the costs of development of any such product candidates or technologies; |
| · | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| · | variations in our financial results or those of companies that are perceived to be similar to us; |
| · | changes in the structure of healthcare payment systems; |
| · | market conditions in the pharmaceutical and biotechnology sectors; |
| · | general economic, industry and market conditions; and |
| · | the other factors described in this “Risk Factors” section. |
| 55 |
In the past, following periods of volatility in the market price of a company’s securities, securities class-action litigation has often been instituted against that company. We also may face securities class-action litigation if we cannot obtain regulatory approvals for or if we otherwise fail to commercialize EGP-437. Such litigation, if instituted against us, could cause us to incur substantial costs to defend such claims and divert management’s attention and resources.
We have received notices from NASDAQ of non-compliance with its continuing listing rules.
On November 20, 2017, we received a notice from NASDAQ notifying us that as of November 20, 2017, we were not in compliance with NASDAQ Listing Rule 5550(b)(1), as we did not maintain a minimum required stockholders’ equity of $2.5 million, or NASDAQ Listing Rule 5550(b)(2), as the market value of our listed securities (“MVLS”) was below the minimum $35 million for the previous 30 consecutive business days, or NASDAQ Listing Rule 5550(b)(3), as we had not had net income from continuing operations in the latest fiscal year or in two of the last three fiscal years. In accordance with NASDAQ Listing Rule 5810(c)(2)(A)(i), we submitted a plan to regain compliance to NASDAQ on January 4, 2018. NASDAQ accepted that plan, and we have a period of 180 calendar days from receipt of the original notice, or until May 21, 2018, to regain compliance. To regain compliance, at any time during the 180 calendar day-compliance period our MVLS must close at $35 million or more for a minimum of 10 consecutive business days or we must report stockholders’ equity of at least $2.5 million. We are actively monitoring our MVLS and stockholders’ equity between now and May 21, 2018, and will consider available options to resolve the deficiency and regain compliance with Rule 5550(b).
In the event that we do not regain compliance with either Listing Rule 5550(b)(1) or Listing Rule 5550(b)(2) prior to the expiration of the compliance period, we will receive written notification that our securities are subject to delisting. At that time, we may appeal the delisting determination to a hearings panel pursuant to the procedures set forth in the applicable NASDAQ Listing Rules. A delisting of our common stock would have an adverse effect on the market liquidity of our common stock and, as a result, the market price for our common stock could become more volatile. Further, a delisting also could make it more difficult for us to raise additional capital.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
As of December 31, 2017, we had federal net operating loss carryforwards of approximately $45.5 million, state net operating loss carryforwards of approximately $33.3 million and aggregate federal and state research and development tax credit carryforwards of approximately $1.6 million and $0.5 million available to reduce future taxable income. These federal and state net operating loss carryforwards and federal and state tax credit carryforwards which will expire at various dates through 2037, if not utilized. Utilization of these net operating loss and tax credit carryforwards may be subject to a substantial limitation under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, and comparable provisions of state, local and foreign tax laws due to changes in ownership of our company that have occurred previously or that could occur in the future. Under Section 382 of the Code and comparable provisions of state, local and foreign tax laws, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes, such as research and development tax credits, to reduce its post-change income may be limited. We have not completed a study to determine whether our initial public offering, our registered direct offering, our follow-on public offerings, and other transactions that have occurred over the past three years may have triggered an ownership change limitation. We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership. As a result, if we generate taxable income, our ability to use our pre-change net operating loss and tax credits carryforwards to reduce U.S. federal and state taxable income may be subject to limitations, which could result in increased future tax liability to us. In addition, the Tax Cuts and Jobs Act (“TCJA”) enacted on December 22, 2017 limits the amount of net operating losses that we are permitted to deduct in any taxable year to 80% of our taxable income in such year. The TCJA also eliminates the ability to carry back net operating losses to prior years, but allows net operating losses generated after 2017 to be carried forward indefinitely. As such, there is a risk that due to such items, our existing net operating losses could expire or be unavailable to offset future income.
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could significantly reduce the market price of our common stock and impair our ability to raise adequate capital through the sale of additional equity securities.
We are an “emerging growth company,” and a smaller reporting company and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and may remain an emerging growth company for up to five years following the end of the fiscal year during which we first sold common equity securities under an effective registration statement, which period for us would end on December 31, 2020. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
| 56 |
| · | being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| · | not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting; |
| · | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
| · | reduced disclosure obligations regarding executive compensation; and |
| · | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We have taken advantage of certain reduced reporting. In particular, in this Annual Report on Form 10-K, we have provided only two years of audited financial statements under the smaller reporting company requirements and have not included all of the executive compensation related information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In addition, the JOBS Act also provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to delay such adoption of new or revised accounting standards, and, as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for public companies that are not emerging growth companies.
We are also a “smaller reporting company” as defined in Rule 12b-2 of the Exchange Act and have elected certain scaled disclosure available for smaller reporting companies.
We incur increased costs as a result of operating as a public company, and our management is required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we are no longer an emerging growth company, we incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, FINRA rules and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly.
We are evaluating these rules and regulations, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404 of the Sarbanes-Oxley Act, or Section 404, we are required to furnish a report by our management on our internal control over financial reporting. However, while we remain either a smaller reporting company and/or an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we engaged in a process to document and evaluate our internal control over financial reporting. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. If we identify one or more material weaknesses in our internal control over financial reporting, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
| 57 |
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, any future debt agreements that we may enter into, may preclude us from paying dividends without the lenders’ consent or at all. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
| Item 1B. | Unresolved Staff Comments. |
None.
| Item 2. | Properties. |
We currently have two facilities including our principal executive office located at 271 Waverley Oaks Road, Suite 108, Waltham, MA 02452, and our office located at 391 Chipeta Way, Suite H, Salt Lake City UT, 84108. We conduct our operations using third-party manufacturing facilities and trial sites. We believe our current facilities are adequate for our needs for the foreseeable future.
| Item 3. | Legal Proceedings. |
While we are not currently a party to any legal proceedings, from time to time we may be a party to a variety of legal proceedings that arise in the normal course of our business.
| Item 4. | Mine Safety Disclosures. |
Not Applicable.
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Price Range of Common Stock
Our common stock began trading on the OTCQB on February 13, 2015 in connection with out IPO, and currently trades under the symbol “EYEG.” Prior to that time, there was no established public trading market for our common stock. On July 31, 2015, our Common Stock and Warrants issued in our follow-on offering, which closed on August 5, 2015, began trading on The NASDAQ Capital Market under the symbols “EYEG” and “EYEGW,” respectively. In connection with this listing, the Common Stock ceased being quoted on the OTCQB Venture Marketplace.
The following table sets forth the range of the high and low sales prices per share of our common stock as reported on the NASDAQ Capital Market and the OTCQB for the quarterly periods indicated. The quotations for periods when our common stock traded on the OTCQB reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
| 58 |
| Fiscal Year Ended December 31, 2017 | High | Low | ||||||
| First Quarter | $ | 3.90 | $ | 1.42 | ||||
| Second Quarter | 2.53 | 1.21 | ||||||
| Third Quarter | 1.47 | 0.90 | ||||||
| Fourth Quarter | $ | 1.38 | $ | 0.99 | ||||
| Fiscal Year Ended December 31, 2016 | High | Low | ||||||
| First Quarter | $ | 4.11 | $ | 1.59 | ||||
| Second Quarter | 3.75 | 2.52 | ||||||
| Third Quarter | 2.56 | 1.46 | ||||||
| Fourth Quarter | $ | 1.85 | $ | 1.26 | ||||
On February 28, 2018, the closing sale price of our common stock on the NASDAQ Market was $0.57 per share. There were 65 holders of record of our common stock as of February 28, 2018. This number does not include beneficial owners whose shares were held in street name.
Dividend Policy
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain future earnings, if any, and all currently available funds for use in the operation of our business and do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors the board of directors deems relevant, and subject to the restrictions contained in our current or future financing instruments.
Recent Sales of Unregistered Securities
None.
| Item 6. | Selected Financial Data |
Not Applicable.
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
Forward-Looking Statements
The following section of this Annual Report on Form 10-K entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contains statements that are not statements of historical fact and are forward-looking statements within the meaning of federal securities laws. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Factors that may cause our actual results to differ materially from those in the forward-looking statements include those factors described in “Item 1A. Risk Factors” beginning on page 25 of this Annual Report on Form 10-K. You should carefully review all of these factors, as well as the comprehensive discussion of forward-looking statements on page 1 of this Annual Report on Form 10-K.
| 59 |
Business Overview
We are a clinical-stage specialty pharmaceutical company focused on developing and commercializing products for treating diseases and disorders of the eye. We accomplish this by leveraging our two proprietary platform technologies, crosslinked thiolated carboxymethyl hyaluronic acid (“CMHA-S”) and iontophoresis drug delivery system. Our CMHA-S platform is based on a modified form of the natural polymer hyaluronic acid (“HA”), which is a gel that possesses unique physical and chemical properties such as hydrating and promoting wound healing when applied to the ocular surface. We believe that the ability of CMHA-S to adhere longer to the ocular surface, while hydrating and promoting wound healing, makes it well-suited for treating various ocular surface injuries from dry eye to corneal wounds.
Hyaluronic acid is a naturally occurring polymer that is important in many physiological processes, including wound healing, tissue homeostasis, and joint lubrication. To create this hydrogel, the HA is modified to create CMHA that is then crosslinked together through the thiol groups to CMHA-S. Crosslinking slows degradation of the HA backbone and provides a matrix for incorporating therapeutic agents. Variations in the number of thiols per molecule, the molecular weight of the polymer, the concentration of the polymer, the type of crosslinking, and incorporation of active ingredients, provides a highly versatile platform that can be tailored to a specific application and formulated as eye drops, gels, or films.
Our first CMHA-S-based product candidate, EyeGate OBG, is a topically applied 0.75% CMHA-S eye drop formulation that has completed its first-in-man or proof-of concept clinical trial. Preclinical studies suggest that the specific CMHA-S chemical modification comprising EyeGate OBG creates a favorable set of attributes, including prolonged retention time on the ocular surface, and a smooth continuous clear barrier without blur that can minimize mechanical lid friction, reduce repeat injury, and mechanically protect the ocular surface, allowing accelerated corneal re-epithelization. It is intended for the management of corneal epithelial wounds/defects and epitheliopathies, and to accelerate re-epithelization of the ocular surface following surgery, infections, and other traumatic and non-traumatic conditions.
EyeGate OBG is being developed pursuant to a de novo 510(k) regulatory pathway for devices submitted for marketing clearance to the U.S. Food and Drug Administration, or FDA. We plan to develop EyeGate OBG for two indications, acceleration of corneal re-epithelization post photorefractive keratectomy and for the reduction of corneal staining in patients with punctate epitheliopathies (i.e. moderate dry eye patients). We believe that EyeGate OBG is the first and only eye drop being developed in the U.S. to target acceleration of corneal re-epithelization.
EyeGate OBG has successfully completed its first-in-man clinical trial demonstrating the acceleration of re-epithelization of the cornea following photorefractive keratectomy. We anticipate approval of our Investigative Device Exemption (IDE) and initiating a second trial, the pilot trial, in the first half of 2018. We plan to file an additional IDE for the same product to treat patients with punctate epitheliopathies, focused on moderate dry eye, in the first quarter of 2018. We anticipate initiating the trial in the first half of 2018.
The same crosslinked HA in EyeGate OBG is presently available commercially as a veterinary device indicated for use in the management of superficial noninfectious corneal ulcers. Manufactured by SentrX Animal Care and sold in the U.S. by Bayer Animal Health as Remend® Corneal Repair, the product has been used successfully for five years in dogs, cats and horses, without adverse effects. The composition of the veterinary product is identical to that of the EyeGate OBG. We have obtained a license from BioTime, Inc. for the exclusive worldwide right to commercialize CMHA-S for ophthalmic treatments in humans. We paid BioTime $50,000, and are required to pay an annual fee of $30,000 and royalties to BioTime based on revenue relating to any product incorporating the CMHA-S technology. Our license agreement expires when patent protection for the CMHA-S technology lapses, which is expected to occur in the U.S. in 2027. We do not have the rights to the CMHA-S platform for animal health or veterinary medicine.
Our other product candidate from our second platform is EGP-437, a reformulated topically active corticosteroid, dexamethasone phosphate, delivered into the ocular tissues through our proprietary innovative iontophoresis drug delivery system, the EyeGate® II Delivery System. The EyeGate® II Delivery System features a compact and easy-to-use device that we believe has the potential to deliver drugs non-invasively and quickly into the ocular tissues through the use of iontophoresis, which can accelerate the onset of action, dramatically reduce dosing frequency compared to regular eye drops, and sustain the duration of therapeutic effect. Iontophoresis employs the use of a low electrical current that promotes the migration of a charged drug substance across biological membranes. The EyeGate® II Delivery System is easy-to-use, taking only a few minutes to deliver medication. More than 3,000 treatments have been administered to date using our EyeGate® II Delivery System in clinical trials. EGP-437 is currently in clinical development for the treatment of various inflammatory conditions of the eye. Current programs include the treatment of ocular inflammation and pain in post-surgical cataract patients and the treatment of uveitis, a debilitating form of intraocular inflammation of the anterior portion of the uvea, such as the iris and/or ciliary body, with a Phase 3 trial currently enrolling. We expect to report top-line data for the uveitis trial in the third quarter of 2018. We announced topline data for the Phase 2b cataract surgery trial in the first quarter of 2018. Although EGP-437 demonstrated a higher rate of success compared to vehicle at all time points, the co-primary endpoints of proportion of subjects with an anterior chamber cell (ACC) count of zero at day 7 and the proportion of subjects with a pain score of zero at day 1 did not show statistical significance. The efficacy results for the absence of inflammatory cells in the EGP-437 treatment group met our expectations, but the vehicle group response was better than anticipated. We will continue to review the data to determine next steps and to continue evaluating EGP-437 for the reduction of pain and inflammation following ocular surgery.
| 60 |
EGP-437 is being developed pursuant to a new drug application, or NDA, under the Section 505(b)(2) pathway, which enables an applicant to rely, in part, on the FDA’s findings of safety and efficacy for an existing product, or published literature, in support of its NDA. In the case of EGP-437, the existing reference product is dexamethasone eye drops. Based on guidance provided by the FDA, we believe that if the planned confirmatory Phase 3 trial of EGP-437 in anterior uveitis meets non-inferiority criteria, the results of that trial, along with data from our previously completed Phase 3 trial in anterior uveitis, will be sufficient to support a NDA filing in the second half of 2018. We also believe, based on guidance provided by the FDA, that the design of the ongoing confirmatory Phase 3 anterior uveitis trial is acceptable and that the nonclinical work completed to date is sufficient to support a NDA filing.
Medical products containing a combination of new drugs, biological products, or medical devices may be regulated as “combination products” in the U.S. A combination product generally is defined as a product comprised of components from two or more regulatory categories, such as drug/device, device/biologic, or drug/biologic. Each component of a combination product is subject to the requirements established by the FDA for that type of component, whether a new drug, biologic, or device. In order to facilitate premarket review of combination products, the FDA designates one of its centers to have primary jurisdiction for the premarket review and regulation of both components. We expect that the Center for Drug Evaluation and Research will have primary jurisdiction over our EGP-437 combination product. The determination whether a product is a combination product or two separate products is made by the FDA on a case-by-case basis. We have had discussions with the FDA about the status of our EGP-437 combination product as a combination product and we have been advised that the FDA considers our product a combination drug/device.
We have entered into two exclusive global license agreements with subsidiaries of Valeant Pharmaceuticals International, Inc. (“Valeant”), through which we have granted Valeant exclusive, worldwide commercial and manufacturing rights to the combination of our EyeGate® II Delivery System and our EGP-437 product in the fields of uveitis and ocular iontophoretic treatment for post-operative ocular inflammation and pain in ocular surgery patients, as well as a right of last negotiation to license the combination product for other indications. We are responsible for the clinical development of the product in the U.S. for the indications licensed, together with the costs associated therewith. Valeant has the right to develop the product in the fields outside of the U.S. and has agreed to fund 100% of any costs associated therewith.
Throughout our history, we have not generated significant revenue. We have never been profitable, and from inception through December 31, 2017, our losses from operations have aggregated $91.8 million. Our Net Loss was approximately $13.2 million and $13.3 million for the twelve months ended December 31, 2017 and 2016, respectively. We expect to incur significant expenses and increasing operating losses for the foreseeable future as we continue the development and clinical trials of and seek regulatory approval for our EGP-437 Product for the treatment of uveitis as well as other indications, and the EyeGate OBG, our lead product candidate for corneal epithelial defects, and any other product candidates we advance to clinical development. If we obtain regulatory approval for EyeGate OBG, we expect to incur significant expenses in order to create an infrastructure to support the commercialization of EyeGate OBG including sales, marketing and distribution functions.
We will need additional financing to support our continuing operations. We will seek to fund our operations through public or private equity, debt financings, license and development agreements, or other sources, which may include collaborations with third parties. Adequate additional financing may not be available to us on acceptable terms, or at all. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy. These conditions raise substantial doubt about our ability to continue as a going concern. We will need to generate significant revenue to achieve profitability, and we may never do so.
EyeGate Pharmaceuticals, Inc. was formed in Delaware on December 26, 2004. We were originally incorporated in 1998 under the name of Optis France S.A. in Paris, France. At that time, the name of the French corporation was changed to EyeGate Pharma S.A.S. and became a subsidiary of EyeGate Pharmaceuticals, Inc. Jade was formed in Delaware on December 31, 2012. EyeGate Pharma S.A.S. and Jade are wholly-owned subsidiaries of EyeGate Pharmaceuticals, Inc.
| 61 |
Financial Overview
Revenues
To date, we have recognized Collaboration Revenue from several U.S. government grants made to Jade for ocular therapeutic research (collectively, the “U.S. Government Grants”). While we receive cash amounts from Valeant as progress payments toward milestones, these are not yet recorded as Revenue. See Note 2, “Significant Accounting Policies”. We expect to continue to incur significant operating losses as we fund research and clinical trial activities relating to our ocular therapeutic assets, consisting of EGP-437, our iontophoretic delivery technology, and our CMHA-S-based products. There can be no guarantee that the losses incurred to fund these activities will succeed in generating revenue.
Research and Development Expenses
We expense all research and development expenses as they are incurred. Research and development expenses primarily include:
| · | non-clinical development, preclinical research, and clinical trial and regulatory-related costs; |
| · | expenses incurred under agreements with sites and consultants that conduct our clinical trials; |
| · | expenses related to generating, filing, and maintaining intellectual property; and |
| · | employee-related expenses, including salaries, bonuses, benefits, travel and stock-based compensation expense. |
Substantially all of our research and development expenses to date have been incurred in connection with our EGP-437 Combination Product and EyeGate OBG. We expect our research and development expenses to increase for the near future as we advance EGP-437 and EyeGate OBG through clinical development, including the conduct of our planned clinical trials. The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. We are unable to estimate with any certainty the costs we will incur in the continued development of our EGP-437 Combination Product and EyeGate OBG. Clinical development timelines, the probability of success and development costs can differ materially from expectations.
We may never succeed in achieving marketing approval for our product candidate.
The costs of clinical trials may vary significantly over the life of a project owing to, but not limited to, the following:
| · | per patient trial costs; |
| · | the number of sites included in the trials; |
| · | the countries in which the trials are conducted; |
| · | the length of time required to enroll eligible patients; |
| · | the number of patients that participate in the trials; |
| · | the number of doses that patients receive; |
| · | the cost of comparative agents used in trials; |
| · | the drop-out or discontinuation rates of patients; |
| · | potential additional safety monitoring or other studies requested by regulatory agencies; |
| · | the duration of patient follow-up; and |
| · | the efficacy and safety profile of the product candidate. |
We do not expect our product candidates to be commercially available, if at all, for the next several years.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation. Our general and administrative expenses consisted primarily of payroll expenses for our full-time employees. Other general and administrative expenses include professional fees for auditing, tax, patent costs and legal services.
| 62 |
We expect that general and administrative expenses will remain consistent for the near future until commercialization of our CMHA-S based products, which could lead to an increase in these expenses.
Total Other Income (Expense)
Total other income (expense) consists primarily of interest income we earn on interest-bearing accounts, and interest expense incurred on our outstanding financing arrangements.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with accounting principles generally accepted in the United States, or U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the expenses during the reporting periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Our actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our financial statements appearing elsewhere in this Annual Report on Form 10-K, we believe that the following accounting policies are the most critical for fully understanding and evaluating our financial condition and results of operations.
Accrued Research and Development Expenses
As part of the process of preparing financial statements, we are required to estimate and accrue research and development expenses. This process involves the following:
| · | communicating with our applicable personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost; |
| · | estimating and accruing expenses in our financial statements as of each balance sheet date based on facts and circumstances known to us at the time; and |
| · | periodically confirming the accuracy of our estimates with selected service providers and making adjustments, if necessary. |
Examples of estimated research and development expenses that we accrue include:
| · | fees paid to contract research organizations and investigative sites in connection with clinical studies; |
| · | fees paid to contract manufacturing organizations in connection with non-clinical development, preclinical research, and the production of clinical study materials; and |
| 63 |
| · | professional service fees for consulting and related services. |
We base our expense accruals related to non-clinical development, preclinical studies, and clinical trials on our estimates of the services received and efforts expended pursuant to contracts with organizations/consultants that conduct and manage clinical studies on our behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts may depend on many factors, such as the successful enrollment of patients, site initiation and the completion of clinical study milestones. Our service providers invoice us as milestones are achieved and monthly in arrears for services performed. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. To date, we have not experienced significant changes in our estimates of accrued research and development expenses after a reporting period.
However, due to the nature of estimates, we cannot assure you that we will not make changes to our estimates in the future as we become aware of additional information about the status or conduct of our clinical studies and other research activities.
Stock-Based Compensation
We have issued options to purchase our common stock and restricted stock. Stock-based compensation cost is measured at the grant date based on the fair value of the award and is recognized as expense over the requisite service/vesting period. Determining the appropriate fair value model and calculating the fair value of stock-based payment awards require the use of highly subjective assumptions, including the expected life of the stock-based payment awards and stock price volatility.
We estimate the grant date fair value of stock options and the related compensation expense, using the Black-Scholes option valuation model. This option valuation model requires the input of subjective assumptions including: (1) expected life (estimated period of time outstanding) of the options granted, (2) volatility, (3) risk-free rate and (4) dividends. In general, the assumptions used in calculating the fair value of stock-based payment awards represent management’s best estimates, but the estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and we use different assumptions, our stock-based compensation expense could be materially different in the future.
| 64 |
Revenue Recognition
The Valeant Agreements entitle us to initial up-front payments, which we received in 2015 and 2017, and recorded as Deferred Revenue on our Consolidated Balance Sheet, as well as certain additional payments, based on research and development progress and paid over several years. Under the Valeant Agreements, there are research and development milestones or deliverables (“R&D Milestones”), for which we receive additional payments. We receive payments both when we cross certain thresholds on the path to each R&D Milestone (each, a “Progress Payment”), as well as once we finally achieve each R&D Milestone. We are entitled to retain all of these payments once received. As of December 31, 2017, we deferred all Progress Payments and capitalized these payments on our Consolidated Balance Sheet as Deferred Revenue, and we recognize these payments as Revenue once we achieve the R&D Milestone to which the Progress Payment relates. The upfront payments are recognized as Revenue ratably as we complete each of the R&D Milestones, the amount recognized being the amount of the upfront payment times the percentage represented by the proportionate share of fair value of each R&D Milestone relative to the total fair value of the all the R&D Milestones. Accordingly, the Deferred Revenue account on our Consolidated Balance Sheet is reduced as Revenue is recognized in our Consolidated Statement of Operations and Comprehensive Loss. Effective January 1, 2018, we will recognize revenue over-time as performance obligations are met and progress and milestone payments are achieved.
We have received U.S. Government Grant funds from two sources: the U.S. Department of Defense (“DoD”) and the National Science Foundation (“NSF”). We were paid by the DoD after we perform specified, agreed-upon research, and we recorded these grant funds as Revenue as we perform the research. We were generally paid by the NSF every six months, before we perform specified, agreed-upon research. The NSF funds were recorded on the Consolidated Balance Sheet as Deferred Revenue when invoiced, and recognized as Revenue ratably as the research was performed, typically over a six-month period. The U.S. Government Grants from the DOD and NSF have been fully funded as of December 31, 2017.
Recent Accounting Pronouncements
In November 2016, the FASB issued ASU No. 2016-18, Restricted Cash, which clarifies guidance and presentation related to restricted cash in the statement of cash flows, including stating that restricted cash should be included within cash and cash equivalents on the statement of cash flows. The standard is effective for fiscal years beginning after December 15, 2017, with early adoption permitted, and is to be applied retrospectively. We adopted this standard effective with these financial statements. Such adoption did not have a material effect on our financial statements and related disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases (“ASU 2016-02”), which is effective for fiscal years, and interim periods within those years, beginning after December 15, 2018, with early adoption permitted. Under ASU 2016-02, lessees will be required to recognize for all leases at the commencement date a lease liability, which is a lessee’s obligation to make lease payments arising from a lease measured on a discounted basis, and the right-to-use assets, which are asset that represents the lessee’s right to use or control the use of a specified asset for the lease term. We do not expect to early adopt this standard and currently have leases (see Note 11) that will be in place at the effective date. We are currently evaluating the effect that the new guidance will have on our financial statements and related disclosures.
In March 2016, the FASB issued an ASU No. 2016-09, Compensation- Stock Compensation (“ASU 2016-09”), which identifies areas for simplification involving several aspects of accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, an option to recognize gross stock compensation expense with actual forfeitures recognized as they occur, as well as certain classifications on the statement of cash flows. This update is effective for fiscal years beginning after December 15, 2016 including interim periods within that reporting period, with early adoption permitted. We have adopted the provisions of ASU 2016-09 in the first quarter of 2017 and the adoption of this guidance did not have a material impact on our consolidated financial statements. The guidance requires the recognition in the income statement of the income tax effects of vested or settled awards. Further, the guidance requires that the recognition of anticipated tax windfalls/shortfalls be excluded in the calculation of assumed proceeds when applying the treasury stock method. The guidance also allows for the employer to repurchase more of an employee’s shares for tax withholding purposes and not classify the award as a liability that requires valuation on a mark-to-market basis. In addition, the guidance allows for a policy election to account for forfeitures as they occur rather than on an estimated basis.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), as subsequently amended, that outlines a single comprehensive model for entities to use in accounting for revenue arising from contracts with customers and supersedes most recent current revenue recognition guidance, including industry-specific guidance. The core principle of the revenue model is that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance also specifies the accounting for certain incremental costs of obtaining a contract, and costs to fulfill a contract with a customer. Entities have the option of applying either a full retrospective approach to all periods presented, or a modified approach that reflects differences prior to the date of adoption as an adjustment to equity. In April 2015, the FASB deferred the effective date of this guidance until January 1, 2018. We will adopt this standard on January 1, 2018. Our sole revenue is from our Valeant Agreements and U.S. Government Grants.
We completed our implementation analysis, including identification of revenue streams and reviews of customer contracts under ASU 2014-09’s framework. Our analysis included reviewing current accounting policies and practices to identify potential differences that would result from applying the requirements under this new standard. We reviewed our contracts with Valeant. ASU 2014-09 requires increased disclosure, which in turn requires certain new processes. We are opting to use the modified retrospective transition method, meaning the cumulative effect of applying the new guidance will be recognized at the date of initial application as an adjustment to the opening accumulated deficit balance, and thus on January 1, 2018, we will record a reduction to our opening accumulated deficit balance of approximately $9.5 million. We will continue to recognize revenue over time in 2018 as performance obligations are met.
| 65 |
Other Information
Net Operating Loss Carryforwards
As of December 31, 2017, we have federal and state income tax net operating loss (“NOL”) carryovers of approximately $45.5 million and $33.3 million, respectively, which will expire at various dates through 2037. As of December 31, 2017, we also have federal, state and foreign research and development tax credit carryforwards of approximately $1.6 million, $0.5 million, and $25,000, respectively, to offset future income taxes, which expire at various times through 2037.
Utilization of these net operating loss and tax credit carryforwards may be subject to a substantial limitation under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, and comparable provisions of state, local and foreign tax laws due to changes in ownership of our company that have occurred previously or that could occur in the future. Under Section 382 of the Code and comparable provisions of state, local and foreign tax laws, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes, such as research and development tax credits, to reduce its post-change income may be limited. We have not completed a study to determine whether our initial public offering, our registered direct offering, our follow-on public offerings, and other transactions that have occurred over the past three years may have triggered an ownership change limitation. We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership. As a result, if we generate taxable income, our ability to use our pre-change net operating loss and tax credits carryforwards to reduce U.S. federal and state taxable income may be subject to limitations, which could result in increased future tax liability to us. In addition, the Tax Cuts and Jobs Act enacted on December 22, 2017, limits the amount of NOLs that we are permitted to deduct in any taxable year to 80% of our taxable income in such year. The TCJA also eliminates the ability to carry back NOLs to prior years but allows NOLs generated after 2017 to be carried forward indefinitely. As such, there is a risk that due to such items, our existing NOLs could expire or be unavailable to offset future income.
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
We have evaluated the benefits of relying on other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board (“PCAOB”) regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an “emerging growth company” until the earliest of (a) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more, (b) the last day of our fiscal year following the fifth anniversary of the date of the completion of our initial public offering, or December 31, 2020, (c) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years or (d) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
| 66 |
Results of Operations
Comparison of Years Ended December 31, 2017 and 2016
The following table summarizes the results of our operations for the years ended December 31, 2017 and 2016:
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | Change | ||||||||||
| Collaboration Revenue | $ | 407,518 | $ | 669,259 | $ | (261,741 | ) | |||||
| Operating Expenses: | ||||||||||||
| Research and Development | (10,330,349 | ) | (8,422,542 | ) | (1,907,807 | ) | ||||||
| General and Administrative | (4,636,408 | ) | (5,593,563 | ) | 957,155 | |||||||
| Total Operating Expenses | (14,966,757 | ) | (14,016,105 | ) | (950,652 | ) | ||||||
| Other (Expense) Income, Net | (651 | ) | 3,409 | (4,060 | ) | |||||||
| Loss Before Income Tax Benefit | (14,559,890 | ) | (13,343,437 | ) | (1,216,453 | ) | ||||||
| Income Tax Benefit | 1,341,973 | - | 1,341,973 | |||||||||
| Net Loss | $ | (13,217,917 | ) | $ | (13,343,437 | ) | $ | 125,520 | ||||
Collaboration Revenue. Collaboration Revenue was $0.408 million for the year ended December 31, 2017, compared to $0.669 million for the year ended December 31, 2016, reflecting the Collaboration Revenue we generate from the U.S. Government Grants in accordance with our contracted agreements. These grants were fully funded as of December 31, 2017.
Research and Development Expenses. Research and Development Expenses were $10.330 million for the year ended December 31, 2017 compared to $8.423 million for the year ended December 31, 2016. The increase of $1.908 million was primarily due to increases in clinical and other activity related to the Phase 2b trial for post-cataract surgery inflammation and pain, the EyeGate OBG, as well as personnel related costs from the expansion of operations following the Jade Acquisition in the first quarter of 2016. These increases were partially offset by a decrease in clinical activity related to the EGP-437 Phase 3 trial for the treatment of anterior uveitis.
General and Administrative Expenses. General and Administrative Expenses were $4.636 million for the year ended December 31, 2017, compared to $5.594 million for the year ended December 31, 2016. The decrease of $0.957 million was mainly due to decreases in professional fees, including costs incurred during the first quarter of 2016 related to the Jade Acquisition.
Income Tax Benefit. Income Tax Benefit was $1.342 million for the year ended December 31, 2017, compared to zero for the year ended December 31, 2016. The increase of $1.342 million was due to the Company’s partial release of its valuation allowance against its previously recorded deferred tax assets as a result of the impact of the Tax Cuts and Jobs Act where future reversals of deductible temporary differences, such as those from the Company’s indefinite-lived in-process research and development, can offset taxable temporary differences from future net operating loss carryforwards due to their indefinite carryforward period under the new tax law.
| 67 |
Liquidity and Capital Resources
Since becoming a public company in 2015, we have financed our operations from four registered offerings of our Common Stock and Convertible Preferred Stock, payments from our Valeant License Agreements and the U.S. Government Grants, and sales through our At The Market Offering Agreement. From inception through December 31, 2017, we have raised a total of approximately $84.5 million from such sales of our equity and debt securities, both as a public company and prior to our IPO, as well as approximately $13.4 million in payments received under our license agreements and U.S. Government Grants.
On February 21, 2017, we received the initial $4.0 million upfront payment from Valeant as provided under the New Valeant Agreement related to our EGP-437 Product in the field of ocular iontophoretic treatment for post-operative ocular inflammation and pain in ocular surgery patients. Through December 31, 2017, we have received cash payments of $12.3 million under the Valeant Agreements, which are presented as Deferred Revenue on our Consolidated Balance Sheet.
On May 24, 2016, we entered into an At The Market Offering Agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC (the “Sales Agent”), to create an at the market equity program under which we can from time to time offer and sell up to 1,319,289 shares of its Common Stock through the Sales Agent. Effective as of June 26, 2016, we halted indefinitely all future offers and sales of our Common Stock pursuant to the ATM Agreement. On June 30, 2016, we closed on the sale of our equity securities in connection with a registered direct offering, described below, and as a result, we were restricted from issuing any shares pursuant to the ATM Agreement for a period of 90 days following June 30, 2016. This restriction lapsed on September 28, 2016. On February 21, 2017, we authorized the Sales Agent to restart sales under the ATM Agreement for maximum aggregate proceeds of up to $3,285,798. During the first quarter of 2017, we sold 642,150 shares of Common Stock under this agreement for total net proceeds to us from this offering, after deducting the placement agent fees and offering expenses, of approximately $1.8 million. We did not sell any shares of Common Stock pursuant to the ATM Agreement during the second, third or fourth quarters of 2017. On June 14, 2017, we closed on the sale of our equity securities in connection with a public offering, described below, and as a result, we are restricted from issuing any shares pursuant to the ATM Agreement for a period of twenty-four months following the closing date of the offering. However, this restriction is suspended for any sale of shares of Common Stock under the ATM Agreement that is above $3.00 per share.
On June 14, 2017, we completed a public offering of 5,336,667 shares of Common Stock and 1,995 shares of Series B Preferred Stock (convertible into 1,330,000 shares of Common Stock), along with warrants to purchase 6,666,667 shares of Common Stock. The total net proceeds to us from this offering, after deducting the placement agent fees and offering expenses, were approximately $8.8 million. As of December 31, 2017, a holder of the Series B Preferred Stock had converted 1,395 shares of Series B Preferred Stock into an aggregate of 930,000 shares of Common Stock.
At December 31, 2017, we had cash and cash equivalents totaling $7,806,029.
The following table sets forth the primary sources and uses of cash for the years ended December 31, 2017 and 2016:
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Net Cash Used in Operating Activities | $ | (6,468,541 | ) | $ | (8,413,180 | ) | ||
| Net Cash (Used In) Provided by Investing Activities | (36,999 | ) | 174,746 | |||||
| Net Cash Provided by Financing Activities | 10,671,353 | 3,498,227 | ||||||
Comparison of Years Ended December 31, 2017 and 2016
Operating Activities. Net cash used in operating activities was $6.469 million for the year ended December 31, 2017, compared to $8.413 million for the year ended December 31, 2016. The primary use of Cash was to fund operating losses of $13.218 million and $13.343 million in 2017 and 2016, respectively, offset by the positive impact of receiving cash payments from Valeant of $8.089 million and $3.225 million in 2017 and 2016, respectively, as well as the U.S. Government, some of which is classified as Deferred Revenue on the Consolidated Balance Sheet, and some of which is included in Collaboration Revenue in the Consolidated Statement of Operations and Comprehensive Loss.
Investing Activities. Net cash (used in) provided by investing activities was $(0.037) million for the year ended December 31, 2017, compared to $0.175 million for the year ended December 31, 2016. During the year ended December 31, 2017, we purchased $0.037 million in office furniture and leasehold improvements. On March 7, 2016, we acquired Jade Therapeutics, Inc., in exchange for shares of our Common Stock and Cash, which required the use of $0.186 million in cash (net of cash acquired).
Financing Activities. We received $10.671 million in cash from financing activities for the twelve months ended December 31, 2017, compared to $3.498 million for the year ended December 31, 2016. This increase of $7.173 million was mainly due to net proceeds received from sales under our ATM Agreement of $1.824 million, as well as net proceeds received from our public offering of $8.765 million, compared to net proceeds of $3.445 million received from our registered direct offering in 2016.
| 68 |
Funding Requirements and Other Liquidity Matters
Our EGP-437 Combination Product and our CMHA-S-based product pipeline are still in various stages of clinical development. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we:
| · | seek marketing approval for our EGP-437 Combination Product and our CMHA-S-based products; |
| · | establish a sales and marketing infrastructure to commercialize our CMHA-S-based products in the United States, if approved; and |
| · | add operational, financial and management information systems and personnel, including personnel to support our product development and future commercialization efforts. |
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our Stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of a Common Stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or licensing arrangements with pharmaceutical partners, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates, including our EGP-437 Product and our CMHA-S-based products, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market the EGP-437 Product and CMHA-S-based products that we would otherwise prefer to develop and market ourselves.
Based on our cash on hand at December 31, 2017 and cash we expect to receive over the first half of 2018, we believe we will have sufficient cash to fund planned operations for approximately seven months. However, the acceleration or reduction of cash outflows by management can significantly impact the timing for raising additional capital to complete development of its products. To continue development, we will need to raise additional capital through debt and/or equity financing, or access additional funding through grants. Although we completed the IPO, a registered direct offering, two follow-on public offerings, and sales under the ATM Agreement, additional capital may not be available on terms favorable to us, if at all. On May 6, 2016, the SEC declared effective our registration statement on Form S-3, registering a total of $100,000,000 of our securities for sale to the public in what is known as a “shelf offering”. We do not know if our future offerings pursuant to our shelf registration statement will succeed. Accordingly, no assurances can be given that management will be successful in these endeavors. Our recurring losses from operations have caused management to determine there is substantial doubt about our ability to continue as a going concern. Our Consolidated Financial Statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities or any other adjustments that might be necessary should we be unable to continue as a going concern.
Off-Balance Sheet Arrangements
We had no material off-balance sheet arrangements at December 31, 2017.
| 69 |
Contractual Obligations and Commitments
The following table summarizes our contractual obligations as of December 31, 2017:
| Total | Less Than 1 Year | 1-3 Years | 3 Years & Thereafter | |||||||||||||
| Leases (1) | $ | 331,947 | $ | 182,940 | $ | 149,007 | $ | - | ||||||||
| Licensing Agreement (2) | 252,500 | 52,500 | 105,000 | 95,000 | ||||||||||||
| Purchase Obligations (3) | 760,450 | 760,450 | - | - | ||||||||||||
| Total (4) | $ | 1,344,897 | $ | 995,890 | $ | 254,007 | $ | 95,000 | ||||||||
| (1) | Lease obligations reflect our obligation to make payments in connection with operating leases for our office space and capital leases with respect to laboratory equipment. |
| (2) | Licensing Agreement obligations represent our commitments under license agreements, including those made by us under our license agreements with the University of Miami School of Medicine, the University of Utah Research Foundation, and BioTime. |
| (3) | Purchase Obligations relate to a Master Service Agreement with a contract research organization (“CRO”). The CRO will provide clinical research services for Phase 3 trials in patients with non-infectious anterior segment uveitis. |
| (4) | This table does not include (a) anticipated expenditures under supply agreements for periods for which we are not yet bound under binding purchase orders, and (b) contracts that are entered into in the ordinary course of business that are not material in the aggregate in any period presented above. |
In addition, in the course of normal business operations, we have agreements with contract service providers to assist in the performance of our research and development and manufacturing activities. Expenditures to contract research organizations vary based on the study and phases during the clinical development stages. Subject to required notice periods and our obligations under binding purchase orders, we can elect to discontinue the work under these agreements at any time. We could also enter into additional collaborative research, contract research, manufacturing, and supplier agreements in the future, which may require upfront payments and even long-term commitments of cash.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk. |
Not Applicable.
| 70 |
| Item 8. | Financial Statements and Supplementary Data. |
The information required by this item is contained in the Consolidated Financial Statements filed as part of this Annual Report on Form 10-K are listed under Item 15 of Part IV below.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None.
| Item 9A. | Controls and Procedures. |
This Report includes the certifications of our President and Chief Executive Officer (who is our principal executive officer) and our Chief Financial Officer (who is our principal financial and accounting officer) required by Rule 13a-14 of the Exchange Act. See Exhibits 31.1 and 31.2. This Item 9A includes information concerning the controls and control evaluations referred to in those certifications.
| (a) | Evaluation of Disclosure Controls and Procedures |
Disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) are designed to ensure that information required to be disclosed in reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms and that such information is accumulated and communicated to management, including the President and Chief Executive Officer, to allow timely decisions regarding required disclosures.
In connection with the preparation of this Annual Report on the Form 10-K, the Company’s Management, under the supervision of, and with the participation of, our President and Chief Executive Officer and our Chief Financial Officer, conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2017. Our disclosure controls and procedures are designed to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and our management necessarily was required to apply its judgment in evaluating and implementing our disclosure controls and procedures. Based upon the evaluation described above, our President and Chief Executive Officer and our Chief Financial Officer have concluded that they believe that our disclosure controls and procedures were effective as of the end of the period covered by this report.
| (b) | Management’s Annual Report on Internal Control Over Financial Reporting |
Our management, under the supervision of our President and Chief Executive Officer and our Chief Financial Officer, is responsible for establishing and maintaining an adequate system of internal control over financial reporting. Internal control over financial reporting (as defined in Rules 13a-15(f) and 15d(f) under the Exchange Act) is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”).
A company’s internal control over financial reporting includes those policies and procedures that: (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (b) provide reasonable assurance that transactions are recorded as necessary to permit preparation of Consolidated Financial Statements in accordance with U.S. GAAP; (c) provide reasonable assurance that receipts and expenditures are being made only in accordance with appropriate authorization of management and the board of directors; and (d) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the Consolidated Financial Statements.
In connection with the preparation of this report, our management conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2017 based on the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Additionally, the adoption of ASU 2014-09 does not have a material impact on our internal control over financial accounting and reporting as a result of (i) the adoption, (ii) the implementation on a going forward basis, and (iii) developing information for disclosure. As a result of that evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2017.
As a smaller reporting company and an “emerging growth company” under the Jumpstart Our Business Startups Act, we are exempt from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002. As a result, EisnerAmper LLP, our independent registered public accounting firm, has not audited or issued an attestation report with respect to the effectiveness of our internal control over financial reporting as of December 31, 2017.
| 71 |
| (c) | Changes in Internal Controls Over Financial Reporting |
Our management, with the participation of the Chief Executive Officer and the Chief Financial Officer, has evaluated whether any change in our internal control over financial reporting occurred during the fourth quarter ended December 31, 2017. Based on that evaluation, management concluded that there were no changes to our internal control over financial accounting and reporting that occurred during the quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial accounting and reporting.
| Item 9B. | Other Information. |
None.
| Item 10. | Directors, Executive Officers and Corporate Governance. |
Executive Officers
Biographical information regarding our executive officers is set forth below. Each executive officer is elected annually by our board and serves until his or her successor is appointed and qualified, or until such individual’s earlier resignation or removal.
| Name | Age | Position | ||
| Stephen From | 54 | Chief Executive Officer and President | ||
| Barbara Wirostko | 52 | Chief Medical Officer | ||
| Michael Manzo | 58 | Vice President of Engineering |
Stephen From, President and Chief Executive Officer — Please refer to “— Directors” below for Mr. From’s biographical information.
Barbara Wirostko, Chief Medical Officer, was a co-founder of Jade, is a board-certified ophthalmologist and a fellow of the American Academy of Ophthalmology. Since 2010, she has maintained an academic research and clinical practice with the University of Utah, Moran Eye Center, as a Clinical Adjunct Associate Professor in Ophthalmology and as an Adjunct Associate Professor of Bioengineering. She served as a Development Lead at Pfizer from 2006 to 2010, where she led a successful EU regulatory EMA filing for Xalatan in pediatric glaucoma and for which she and her team were recognized. Dr. Wirostko received her undergraduate degree at Cornell University, College of Arts & Sciences in microbiology and her medical degree at Columbia University, College of Physicians & Surgeons. She interned at Hackensack Medical Center in transitional medicine and completed her residency in ophthalmology at Columbia Presbyterian Medical Center. Dr. Wirostko was awarded a fellowship at The New York Hospital Cornell Medical Center in glaucoma.
Michael Manzo, Vice President of Engineering, has been with us since October 2006 and has served as Vice President of Engineering for the last seven years. Mr. Manzo has over 30 years of experience in product development and manufacturing in the medical device industry. Prior to working at EyeGate, Mr. Manzo held positions of President and Chief Operating Officer (2002 – 2006) at Jenline Industries, Ltd., which is now part of Helix Medical, LLC. He has been part of multiple start-up companies over the years, ranging in medical specialties from cardiology, radiology, urology and laproscopic surgery. Mr. Manzo holds a Masters in Business Administration Degree from Suffolk University and a Bachelor of Science Degree in engineering from University of Massachusetts, Lowell.
Directors
Set forth below is certain information regarding the directors of the Company, based on information furnished to the Company by each director. The biographical description below for each director includes his age, all positions he holds with the Company, his principal occupation and business experience over the past five years, and the names of other publicly-held companies for which he currently serves as a director or has served as a director during the past five years. The biographical description below for each director also includes the specific experience, qualifications, attributes and skills that led to the conclusion by the board of directors that such person should serve as a director of the Company. In addition to such specific information, we also believe that all of our directors have a reputation for integrity, honesty and adherence to high ethical standards. Further, they have each demonstrated business acumen and an ability to exercise sound judgment as well as a commitment of service to the Company and our board.
| 72 |
The board of directors has determined that the director nominees and all the incumbent directors listed below are “independent” as such term is currently defined by applicable NASDAQ rules, except for Mr. From who is also an executive officer of the Company.
The following information is current as of February 28, 2018, based on information furnished to the Company by each director:
Directors of EyeGate Pharmaceuticals, Inc.
| Name | Age |
Position with the Company |
Director Since | |||
| Class III Directors – Term expires 2018 | ||||||
| Stephen From | 54 | President, CEO and Director | October 2005 | |||
| Thomas Balland | 40 | Director | September 2012 | |||
| Class I Directors – Term expires 2019 | ||||||
| Paul Chaney(3) | 60 | Chairman | September 2007 | |||
| Bernard Malfroy-Camine(1)(2) | 65 | Director | July 2012 | |||
| Class II Directors – Term expires 2020 | ||||||
| Thomas E. Hancock(1)(3) | 53 | Director | January 2007 | |||
| Praveen Tyle(1)(2)(3) | 57 | Director | June 2008 | |||
| Morton F. Goldberg(2) | 80 | Director | October 2008 |
| (1) | Member of the compensation committee |
| (2) | Member of the nominating and corporate governance committee |
| (3) | Member of the audit committee |
Class III Directors — Term expires 2018
Stephen From, President and Chief Executive Officer, has served as our President, Chief Executive Officer, and director since October 2005. Mr. From was formerly the Chief Financial Officer at Centelion SAS, an independent biotechnology subsidiary of Sanofi-Aventis. Prior to this, Mr. From spent several years as an investment banker specializing in the biotechnology and medical device sectors. He served as Director in the Global Healthcare Corporate and Investment Banking Group and Head of European Life Sciences for Bank of America Securities. Mr. From holds a BSc from the University of Western Ontario, an accounting diploma from Wilfred Laurier University and has qualified as a Chartered Accountant in Ontario, Canada.
We believe Mr. From’s qualifications to sit on our board of directors include his executive leadership experience, financial expertise and the knowledge and understanding he has gained from serving as our President and Chief Executive Officer since 2005.
Thomas Balland, Director, has served as a director since September 2012. He is a Managing Director at IPSA, a venture capital firm, where he has been since 2002. He has over 16 years of venture capital investment experience. In addition to the Company, Mr. Balland has previously invested in and served on the boards of several biotech and medtech companies including CMC Biologics and SpineGuard. He was also on the boards of several companies that were acquired by larger entities in the life sciences industry, including Technolas Perfect Vision GmbH. Prior to joining IPSA in 2002, Mr. Balland held various positions with firms such as Mars, Inc. and Up&Up. He has degrees in engineering and finance from INSA Lyon and ESCP-EAP, respectively.
We believe Mr. Balland’s qualifications to sit on our board of directors include his executive leadership experience and his business development, strategic planning and mergers and acquisitions experience with biotech and medtech companies.
| 73 |
Class I Directors — Term Expires in 2019
Paul Chaney, Chairman of the Board, has served as a director since September 2007. He is co-founder, President & CEO of PanOptica, Inc, a private venture-backed biopharmaceutical company that licenses and develops drugs for the treatment of important ophthalmic conditions, and has held such positions since March 2009. Prior to founding PanOptica, Mr. Chaney was Executive Vice President and President of Eyetech Pharmaceuticals Inc., or Eyetech. Prior to being acquired by OSI Pharmaceuticals Inc., Mr. Chaney served as Eyetech’s Chief Operating Officer, where he was responsible for the launch of Macugen, the first anti-VEGF treatment for neovascular age-related macular degeneration (wet-AMD), and was part of the executive team which led Eyetech’s initial public offering in 2004. Mr. Chaney has over 30 years of experience in the biopharmaceutical and ophthalmic medical device industry, including a variety of senior management positions at Pharmacia Corporation. He began his career as a sales representative for The Upjohn Company in 1980. Mr. Chaney has also served as a member of the board of directors of Eleven Biotherapeutics, Inc., a biologics company focusing on targeted protein therapeutics, since February 2014. Mr. Chaney earned a double BA in English and Biological Sciences from the University of Delaware.
We believe Mr. Chaney’s qualifications to sit on our board of directors include his executive leadership experience, including 20 years leading major ophthalmology businesses both in the U.S. and globally for both a large public pharmaceutical company and privately held start-ups. Mr. Chaney’s responsibilities have spanned commercial operations, manufacturing, regulatory, business development, non-clinical and clinical development functions. He was responsible for building and leading the commercial organizations responsible for the launches of major glaucoma and retina therapeutics, and commercializing the ophthalmic device business for Pharmacia Corporation.
Bernard Malfroy-Camine, PhD, Director, has served as a director since July 2012. He is a scientist-turned-entrepreneur with nearly 30 years of experience in biotechnology and drug discovery. Since May 2013, he has been President and CEO of ViThera Pharmaceuticals, Inc. He has also served as Director, Business Development US Operations at Voisin Consulting, Inc. (also known as Voisin Consulting Life Sciences) since September 2012. Since October 2008, Dr. Malfroy-Camine has also been Founder, President and CEO of MindSet Rx, Inc., a virtual company which is a continuation of Eukarion, Inc., a biotech company he had founded in 1991, and of which he was President and CEO. Dr. Malfroy-Camine has over 80 scientific publications and holds approximately 20 patents. He has a Master’s degree in Mathematics and Physics from Ecole Polytechnique (Paris) and a Ph.D. in Neurobiology from University Paris VI.
We believe Dr. Malfroy-Camine’s qualifications to sit on our board of directors include his executive leadership experience and his extensive experience in entrepreneurship, drug discovery and drug development.
Class II Directors — Term expires 2020
Thomas E. Hancock, Director, has served as a director since January 2007. He has over fifteen years of experience in the biopharmaceutical industry and equity capital markets. Since September 2004, he has been the Principal of Nexus Medical Partners, where he has been responsible for several investments, including A&G Pharmaceuticals Inc., Magellan Biosciences, Inc., and Panacos Pharmaceuticals, Inc. and a principal of Nexus Investment Company, a FINRA member. Prior to joining Nexus Medical Partners, Thomas was a Senior Equity Analyst and Managing Director at US Bancorp Piper Jaffray, covering both the biopharmaceutical and drug discovery tools markets. He has also held numerous positions at Genentech, Inc. and COR Therapeutics, Inc. Mr. Hancock has a BS in Molecular Biology and an MBA from UC Berkeley.
We believe Mr. Hancock’s qualifications to sit on our board of directors include his many years of biotech, investment banking and venture capital experience.
Praveen Tyle, PhD, Director, has served as a director since June 2008. Since May 2016, Dr. Tyle has served as Executive Vice President of Research and Development of Lexicon Pharmaceuticals. Dr. Tyle was previously a member of the executive management team at Osmotica Pharmaceutical Corp., serving as President and Chief Executive Officer from January 2013 through April 2016 and as Executive Vice President and Chief Scientific Officer from August 2012 to December 2012. He is also a member of the board of Orient EuroPharma Co., Ltd. of Taiwan. Dr. Tyle has nearly 30 years of experience in the pharmaceutical industry with the majority of his tenure in senior executive leadership positions in areas of research and development, manufacturing, quality, business development and operations. Prior to joining Osmotica Pharmaceutical Corp., Dr. Tyle served as Executive Vice President (from January 2012 to August 2012) and Chief Scientific Officer (from October 2011 to August 2012) for the United States Pharmacopeia, or USP. Prior to joining USP, Dr. Tyle from 2008 to 2011, served as the Senior Vice President and Global Head of Business Development and Licensing at Novartis Consumer Health from March 2009 to September 2011. At Novartis Consumer Health, Dr. Tyle also served as Senior Vice President & Global Head of Research and Development from March 2009 to February 2010. Dr. Tyle holds a doctorate in pharmaceutics and pharmaceutical chemistry from the Ohio State University and a BS in Pharmacy (honors) from the Institute of Technology, Banaras Hindu University in India.
| 74 |
We believe Dr. Tyle’s qualifications to sit on our board of directors include his executive research and development leadership experience and significant mergers and acquisitions and business development and licensing experience.
Morton F. Goldberg, MD, Director, has served as a director since October 2008. Since 2003 he has served as the Joseph E. Green Professor of Ophthalmology at the Wilmer Eye Institute, Johns Hopkins University School of Medicine, to which position he was appointed to in 2003. From 1989 to 2003 he served as the Director and William Holland Wilmer Professor of Ophthalmology at the Wilmer Eye Institute. Prior to this, he was a Professor and Chairman of the Department of Ophthalmology at the University of Illinois College of medicine in Chicago for nearly 20 years. Dr. Goldberg trained at Johns Hopkins as a resident and chief resident, and holds a joint appointment at the Johns Hopkins Applied Physics Laboratory. He is also a past President of the Association for Research in Vision and Ophthalmology, the Macula Society, and the Association of University Professors of Ophthalmology. Dr. Goldberg received his undergraduate degree with honors from Harvard College and his MD with honors from Harvard Medical School.
We believe Dr. Goldberg’s qualifications to sit on our board of directors include his extensive expertise in eye care. He is a board certified in ophthalmology and highly experienced in both research and clinical ophthalmology. He has served as academic department chairman for almost 40 years, and also served as Chief Editor of the Archives of Ophthalmology, an important scientific and clinical journal. He has recently completed 50 years of personal eye research as well as personal care of innumerable eye patients having diseases amenable to treatment by iontophoresis.
Section 16(a) Beneficial Ownership Reporting Compliance
Our executive officers, directors and beneficial owners of more than 10% of our Common Stock are required under Section 16(a) of the Securities Exchange Act of 1934 to file reports of ownership and changes in ownership with the Securities and Exchange Commission. Copies of those reports must also be furnished to us.
Based solely on a review of the copies of the reports furnished to us, and written representations from certain reporting persons that no other reports were required, we believe that during the year ended December 31, 2017, the reporting persons complied on a timely basis with all Section 16(a) filing requirements applicable to them, except that due to administrative error, Ventech Capital II filed a late Form 4 on March 21, 2017 relating to shares of common stock sold on March 13, 2017, and Michael Garanzini, the Company’s Chief Commercial Officer, filed a late Form 3 on November 15, 2017 reflecting his appointment to that position on November 1, 2017.
Code of Business Conduct
Our board of directors have adopted a code of business conduct that applies to each of our directors, officers and employees. The code addresses various topics, including:
| · | compliance with applicable laws, rules and regulations; |
| · | conflicts of interest; |
| · | public disclosure of information; |
| · | insider trading; |
| · | corporate opportunities; |
| · | competition and fair dealing; |
| · | gifts; |
| · | discrimination, harassment and retaliation; |
| · | health and safety; |
| · | record-keeping; |
| · | confidentiality; |
| · | protection and proper use of company assets; |
| · | payments to government personnel; and |
| · | reporting illegal and unethical behavior. |
| 75 |
The code of business conduct is posted on our website. Any waiver of the code of business conduct for an executive officer or director may be granted only by our board of directors or a committee thereof and must be timely disclosed as required by applicable law. The code of business conduct will implement whistleblower procedures that establish format protocols for receiving and handling complaints from employees. Any concerns regarding accounting or auditing matters reported under these procedures will be communicated promptly to the audit committee.
Stockholder Nomination Procedures
As of February 28, 2018, there have been no material changes to the procedures by which stockholders may recommend nominees to our Board of Directors.
Audit Committee
Our board of directors has established an audit committee, which is comprised of Thomas E. Hancock, Paul Chaney and Praveen Tyle, each of whom is a non-employee member of the board of directors. Thomas E. Hancock serves as the chair of the audit committee. The audit committee met four times during 2017. The audit committee’s main function is to oversee our accounting and financial reporting processes, internal systems of control, independent registered public accounting firm relationships and the audits of our financial statements. Pursuant to the audit committee charter, the functions of the committee include, among other things:
| · | appointing, approving the compensation of, and assessing the independence of our registered public accounting firm; |
| · | overseeing the work of our registered public accounting firm, including through the receipt and consideration of reports from such firm; |
| · | reviewing and discussing with management and the registered public accounting firm our annual and quarterly financial statements and related disclosures; |
| · | monitoring our internal control over financial reporting and our disclosure controls and procedures; |
| · | meeting independently with our registered public accounting firm and management; |
| · | preparing the audit committee report required by SEC rules; |
| · | reviewing and approving or ratifying any related person transactions; and |
| · | overseeing our risk assessment and risk management policies. |
All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC. Our board of directors has determined that Thomas E. Hancock is an “audit committee financial expert” as defined by applicable SEC rules. In addition, our board of directors has also determined that Mr. Hancock has the requisite financial sophistication under applicable NASDAQ rules and regulations.
| 76 |
| Item 11. | Executive Compensation. |
We are an “emerging growth company” within the meaning of the Jumpstart Our Business Startups Act of 2012. As a result, we have elected to comply with the reduced disclosure requirements applicable to emerging growth companies in accordance with SEC rules. We had three executive officers during the fiscal year ended December 31, 2017, Stephen From, our President and Chief Executive Officer, Barbara Wirostko, our Chief Medical Officer, and Michael Manzo, our Vice President of Engineering, who are our named executive officers.
Summary Compensation Table
The following table sets forth information concerning the compensation of our named executive officers during our fiscal years ended December 31, 2017 and December 31, 2016.
| Name and Principal Position | Year | Salary ($) |
Bonus ($)(1) |
Stock Awards(2) ($) |
Option Awards(2) ($) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||||||||
| Stephen From, President and | 2017 | 400,000 | 200,000 | 91,200 | 183,750 | — | 874,950 | |||||||||||||||||||||
| Chief Executive Officer | 2016 | 400,000 | 275,000 | — | 246,863 | — | 921,863 | |||||||||||||||||||||
| Barbara Wirostko, | 2017 | 280,000 | 68,811 | 22,800 | 40,600 | — | 412,211 | |||||||||||||||||||||
| Chief Medical Officer | 2016 | 213,692 | — | — | 90,016 | — | 303,708 | |||||||||||||||||||||
| Michael Manzo, | 2017 | 250,000 | 75,000 | — | 55,100 | — | 380,100 | |||||||||||||||||||||
| Vice President of Engineering | 2016 | 250,000 | 105,000 | — | 55,949 | — | 410,949 | |||||||||||||||||||||
| (1) | The amounts in this column represent discretionary bonus payments granted by the board in the applicable fiscal year. |
| (2) | The amounts in this column represent the aggregate grant date fair value of option awards or stock awards granted to the officer in the applicable fiscal year, computed in accordance with FASB ASC Topic 718. See Note 2 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K for a discussion of the assumptions made by us in determining the grant date fair value of our equity awards. In accordance with SEC rules, the grant date fair value of an award subject to performance conditions is based on the probable outcome of the conditions. |
Narrative Disclosure to Compensation Tables
Employment Agreements
Stephen From
We originally entered into an amended and restated employment agreement with our President and Chief Executive Officer, Stephen From, effective as of April 28, 2006. Pursuant to this agreement, Mr. From received an annual base salary of $275,078 and he was entitled to receive a bonus of up to 50% of his annual base salary for the applicable fiscal year, and which was $130,000 for the year ended December 31, 2014.
In February 2016, we entered into a second amended and restated employment agreement with Mr. From that became effective upon our listing on the NASDAQ Capital Market on July 31, 2015. Pursuant to this agreement, Mr. From currently receives an annual base salary of $400,000 and is entitled to receive a bonus of up to 50% of his annual base salary for the applicable fiscal year.
On November 29, 2017, we entered into a Third Amended and Restated Employment Agreement with Mr. From. The restated agreement amended Mr. From’s employment agreement to, among other things, provide for a severance payment to Mr. From upon the occurrence of a Change of Control (as defined in the agreement) of the Company, with the payment amount to be determined based on the value of the transaction that results in the Change of Control, up to a maximum of 1.5% of the transaction value. Additionally, the restated agreement increases the benefits that would be realized by Mr. From upon termination by us without Cause or by Mr. From for Good Reason (as such terms are defined in the restated agreement) to include (i) 18 months of salary continuation payments, (ii) an amount equal to 1.5 multiplied by the performance bonus that would have been received in the year of termination, (iii) 18 months of COBRA subsidy payments, and (iv) 18 months of accelerated vesting of stock options and/or restricted stock awards that are unvested at the time of termination.
| 77 |
Barbara Wirostko
On March 7, 2016, we entered into an offer letter with our Chief Medical Officer, Barbara Wirostko. Pursuant to this letter, Dr. Wirostko receives an annual base salary of $280,000, and she is entitled to receive a bonus of up to 30% of her annual base salary for the applicable fiscal year. Additionally, pursuant to the offer letter, Dr. Wirostko received a stock option to purchase 38,286 shares of our common stock, which will vest based on her continued employment with respect to one-third (1/3) of the underlying shares on the first anniversary of the grant date and ratably in monthly installments over the following 24 months.
Michael Manzo
We originally entered into an offer letter with our Vice President of Engineering, Michael Manzo, effective as of August 24, 2006. Pursuant to this agreement, Mr. Manzo received an annual base salary of $200,000, which was increased from $175,049 by an amendment following our initial public offering, and he is entitled to receive a bonus of up to 15% of his annual base salary for the applicable fiscal year. Mr. Manzo did not receive a bonus for the year ended December 31, 2014.
In July 2014, our board of directors approved an amended and restated offer letter with Mr. Manzo that became effective upon our listing on the NASDAQ Capital Market on July 31, 2015. Pursuant to this letter, Mr. Manzo currently receives an annual base salary of $250,000 and is entitled to receive a bonus of up to 30% of his annual base salary for the applicable fiscal year. This agreement supersedes in its entirety any prior offer letters we had with Mr. Manzo.
Change of Control
Each of our named executive officers is eligible to receive certain benefits in the event of a change in control or if his employment is terminated under certain circumstances, as described under “Potential Payments Upon Termination or Change in Control” below.
Equity Compensation
We grant stock options and restricted shares to our named executive officers as the long-term incentive component of our compensation program. Stock options allow employees to purchase shares of our Common Stock at a price per share equal to the fair market value of our Common Stock on the date of grant and may or may not be intended to qualify as “incentive stock options” for United States federal income tax purposes. In the past, our board of directors has determined the fair market value of our Common Stock based upon inputs including valuation reports prepared by third-party valuation firms. Generally, one third of the equity awards we grant vest on the first year anniversary, with the remainder vesting in equal monthly installments over 24 months, subject to the employee’s continued employment with us on the vesting date and our board of directors has discretion to provide that granted options will vest on an accelerated basis if a change of control of our company occurs, either at the time such award is granted or afterward.
Potential Payments Upon Termination or Change in Control
Stephen From
Pursuant to his employment agreement, if we terminate the employment of Stephen From without Cause or if he resigns for Good Reason, then he will be eligible to receive:
| · | continued payment of base salary for 18 months; |
| · | a lump-sum cash payment equal to his 1.5 multiplied by the target bonus payment for the year in which the termination occurs; and |
| · | payment by us of the monthly premiums under COBRA for Mr. From for up to 18 months following the termination. |
“Cause” means the officer’s unlawful or dishonest conduct, or a breach of any of his obligations made under his employment agreement, including, but to limited to, the confidentiality provisions.
“Good Reason” means a resignation after one of the following conditions has come into existence without the officer’s consent: (i) a material reduction in duties, authority or responsibility; (ii) a material reduction in annual base salary; (iii) a relocation of principal place of employment that increases his one-way commute by more than 50 miles; or (iv) a material breach by us of his employment agreement.
Upon a Change in Control, as defined in Mr. From’s employment agreement, Mr. From would receive a severance payment, with the payment amount to be determined based on the value of the transaction that results in the Change of Control, up to a maximum of 1.5% of the transaction value.
| 78 |
Barbara Wirostko
Pursuant to her offer letter, Dr. Wirostko resigns for Good Reason at any time, or if we terminate the employment of Dr. Wirostko after she has been employed by us for at least one year, including within 12 months following a Change in Control (as defined in Dr. Wirostko’s offer letter), then she will be eligible to receive:
| · | continued payment of base salary for six months; and |
| · | a lump-sum cash payment equal to her target bonus payment for the year in which the termination occurs. |
“Cause” means the officer’s unlawful or dishonest conduct, or a breach of any of her obligations made under her offer letter, including, but to limited to, obligations under a separate agreement relating to inventions, non-competition and non-solicitation.
“Good Reason” means a resignation after one of the following conditions has come into existence without the officer’s consent: (i) a material reduction in duties, authority or responsibility; (ii) a material reduction in annual base salary; (iii) a relocation of principal place of employment that increases her one-way commute by more than 50 miles; or (iv) a material breach by us of her offer letter.
Michael Manzo
Pursuant to his offer letter, if we terminate the employment of Michael Manzo without Cause or if he resigns for Good Reason, then he will be eligible to receive:
| · | continued payment of base salary for six months; and |
| · | a lump-sum cash payment equal to his target bonus payment for the year in which the termination occurs. |
“Cause” means the officer’s unlawful or dishonest conduct, or a breach of any of his obligations made under his offer letter, including, but to limited to, obligations under a separate agreement relating to inventions, non-competition and non-solicitation.
“Good Reason” means a resignation after one of the following conditions has come into existence without the officer’s consent: (i) a material reduction in duties, authority or responsibility; (ii) a material reduction in annual base salary; (iii) a relocation of principal place of employment that increases his one-way commute by more than 50 miles; or (iv) a material breach by us of his offer letter.
Change in Control Severance Plan
On November 27, 2017, we adopted the EyeGate Pharmaceuticals, Inc. Change in Control Severance Plan (the “Change in Control Severance Plan”). The Change in Control Severance Plan provides us with assurance that we will have the continued dedication of, and the availability of objective advice and counsel from, executives and other employees and promotes certainty and minimize potential disruption for our employees in the event we are faced with or undergo a change in control. All of our full-time employees are participants in the Change in Control Severance Plan, with the exception of Mr. From. Under the Change in Control Severance Plan, upon a termination of employment without Cause by us or for Good Reason by the employee (as such terms are defined in the Change in Control Severance Plan), in either case within six months following a Change in Control (as defined in the Change in Control Severance Plan), subject to the execution of a release of claims, our full-time employees (other than Mr. From) would be entitled to the following compensation and benefits:
| · | a lump sum severance payment equal to three weeks of such employee’s then-effective base salary rate for each year of service completed by the employee, subject to the following minimum and maximum amounts: |
| · | for all participants that are executive officers or have the title of vice president or higher, a minimum amount equal to 26 weeks of base salary and a maximum amount equal to 52 weeks of base salary, and |
| · | for all other participants, a minimum amount equal to eight weeks of base salary and a maximum amount equal to 26 weeks of base salary; |
| · | a lump sum payment of the employee’s prorated annual incentive award for the year of termination, determined assuming achievement of target performance; |
| · | the payment of any annual incentive that has been earned but not yet paid in respect of any performance period that has concluded as of the executive officer’s termination of employment; and |
| · | payment of health insurance premiums under COBRA for six months following the date of termination, provided that all such premium payments will cease if the executive officer becomes entitled to receive health insurance coverage under another employer-provided plan. |
In the event that any payments under the plan are subject to Section 280G of the Internal Revenue Code, such payments will be reduced, unless not reducing the amount would result in an after-tax benefit to the employee of at least 5% greater than the reduced amount. The Change in Control Severance Plan does not provide excise tax gross-ups on payments to participants.
| 79 |
Employee Benefits and Perquisites
Our named executive officers are eligible to participate in our health and welfare plans to the same extent as all full-time employees. We do not provide our named executive officers with perquisites or other personal benefits other than reimbursement of their healthcare premiums (prior to our offering health plans), as described in the Summary Compensation Table.
Outstanding Equity Awards at 2017 Fiscal Year-End
The following tables show certain information regarding outstanding equity awards held by our named executive officers as of December 31, 2017.
Generally, one-third of the options and shares of restricted stock granted to our named executive officers vest on the one-year anniversary of grant, with the remaining options or shares, as applicable, vesting monthly for two years thereafter, subject to our repurchase right in the event that the executive’s service terminates before vesting in such shares. For information regarding the vesting acceleration provisions applicable to the options held by our named executive officers, please see “Employment Agreements” above.
Option Awards
| Name | Grant Date | Number of Securities Underlying Unexercised Options (#) Vested |
Number of Securities Underlying Unexercised Options (#) Unvested |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||
| Stephen From | 15-Apr-08 | 27,803 | — | 0.65 | 15-Apr-18 | |||||||||||
| 23-Jan-09 | 2,156 | — | 0.65 | 23-Jan-19 | ||||||||||||
| 23-Jan-09 | 278 | — | 0.65 | 23-Jan-19 | ||||||||||||
| 29-Jan-10 | 54,008 | — | 0.65 | 29-Jan-20 | ||||||||||||
| 25-Jun-10 | 34,672 | — | 0.65 | 25-Jun-20 | ||||||||||||
| 14-Jan-11 | 4,553 | — | 0.65 | 14-Jan-21 | ||||||||||||
| 14-Jan-11 | 47,438 | — | 0.65 | 14-Jan-21 | ||||||||||||
| 23-Dec-12 | 10,928 | — | 0.65 | 23-Dec-22 | ||||||||||||
| 19-Feb-15 | 3,642 | — | 6.00 | 19-Feb-25 | ||||||||||||
| 24-Feb-15 | 167,708 | 7,292 | (2) | 5.75 | 24-Feb-25 | |||||||||||
| 28-Aug-15 | 41,666 | 8,334 | (2) | 3.59 | 28-Aug-25 | |||||||||||
| 25-Jan-16 | 12,777 | 7,223 | (1) | 1.70 | 25-Jan-26 | |||||||||||
| 29-Mar-16 | 31,226 | 22,305 | (1) | 3.05 | 29-Mar-26 | |||||||||||
| 18-Jul-16 | 32,655 | 36,497 | (1) | 2.42 | 18-Jul-26 | |||||||||||
| 18-May-17 | 25,000 | — | 1.80 | 18-May-27 | ||||||||||||
| 21-Jun-17 | — | 125,000 | (1) | 1.35 | 21-Jun-27 | |||||||||||
| Barbara Wirostko | 7-Mar-16 | 22,332 | 15,954 | (1) | 3.80 | 7-Mar-26 | ||||||||||
| 29-Mar-16 | 2,001 | 1,430 | (1) | 3.05 | 29-Mar-26 | |||||||||||
| 18-Jul-16 | 2,156 | 2,410 | (1) | 2.42 | 18-Jul-26 | |||||||||||
| 21-Jun-17 | — | 35,000 | (1) | 1.35 | 21-Jun-27 | |||||||||||
| Michael Manzo | 15-Apr-08 | 3,436 | — | 0.65 | 15-Apr-18 | |||||||||||
| 23-Jan-09 | 268 | — | 0.65 | 23-Jan-19 | ||||||||||||
| 23-Jan-09 | 1,366 | — | 0.65 | 23-Jan-19 | ||||||||||||
| 29-Jan-10 | 6,885 | — | 0.65 | 29-Jan-20 | ||||||||||||
| 25-Jun-10 | 4,567 | — | 0.65 | 25-Jun-20 | ||||||||||||
| 14-Jan-11 | 1,366 | — | 0.65 | 14-Jan-21 | ||||||||||||
| 14-Jan-11 | 6,400 | — | 0.65 | 14-Jan-21 | ||||||||||||
| 23-Dec-12 | 10,928 | — | 0.65 | 23-Dec-22 | ||||||||||||
| 19-Feb-15 | 3,187 | — | 6.00 | 19-Feb-25 | ||||||||||||
| 24-Feb-15 | 47,916 | 2,084 | (2) | 5.75 | 24-Feb-25 | |||||||||||
| 28-Aug-15 | 8,333 | 1,667 | (2) | 3.59 | 28-Aug-25 | |||||||||||
| 25-Jan-16 | 3,194 | 1,806 | (1) | 1.70 | 25-Jan-26 | |||||||||||
| 29-Mar-16 | 7,005 | 5,004 | (1) | 3.05 | 29-Mar-26 | |||||||||||
| 18-Jul-16 | 7,352 | 8,217 | (1) | 2.42 | 18-Jul-26 | |||||||||||
| 06-Feb-17 | — | 15,000 | (1) | 1.52 | 06-Feb-27 | |||||||||||
| 18-May-17 | 8,000 | — | 1.80 | 18-May-27 | ||||||||||||
| 21-Jun-17 | — | 20,000 | (1) | 1.35 | 21-Jun-27 | |||||||||||
| (1) | One-third of these options vest on the one-year anniversary of the grant date, with the remainder vesting in equal monthly installments over the remaining two years. |
| (2) | One-quarter of these options vest as of the grant date, one-quarter vest on the one-year anniversary of the grant date, with the remainder vesting in equal monthly installments over two years. |
All option awards were granted under our 2005 Equity Incentive Plan, or the 2005 Plan, and our 2014 Employee Stock Purchase Plan, or the 2014 Plan.
| 80 |
Unvested Shares of Restricted Stock
| Name | Grant Date |
Number of Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($) |
|||||||
| Stephen From | 06-Feb-17 | 60,000 | (1) | 64,200 | ||||||
| Barbara Wirostko | 06-Feb-17 | 15,000 | (1) | 16,050 | ||||||
| (1) | Represents awards of restricted stock. One-third of these shares will vest, and the restrictions thereon will lapse, on the one-year anniversary of the grant date, with the remainder vesting in equal monthly installments over the remaining two years. |
All shares of restricted stock were granted under our 2014 Plan.
Limitations of Liability and Indemnification Matters
Our amended and restated certificate of incorporation and our amended and restated bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by the Delaware General Corporation Law, which prohibits our amended and restated certificate of incorporation from limiting the liability of our directors for the following:
| · | any breach of the director’s duty of loyalty to us or our stockholders; |
| · | acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| · | unlawful payment of dividends or unlawful stock repurchases or redemptions; or |
| · | any transaction from which the director derived an improper personal benefit. |
Our amended and restated certificate of incorporation and our amended and restated bylaws also provide that if Delaware law is amended to authorize corporate action further eliminating or limiting the personal liability of a director, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law, as so amended. This limitation of liability does not apply to liabilities arising under the federal securities laws and does not affect the availability of equitable remedies such as injunctive relief or rescission.
Our amended and restated certificate of incorporation and our amended and restated bylaws also provide that we shall have the power to indemnify our employees and agents to the fullest extent permitted by law. Our amended and restated bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in this capacity, regardless of whether our amended and restated bylaws would permit indemnification. We have obtained directors’ and officers’ liability insurance.
We entered into separate indemnification agreements with our directors and executive officers, in addition to indemnification provided for in our amended and restated certificate of incorporation and amended and restated bylaws. These agreements, among other things, provide for indemnification of our directors and executive officers for certain expenses, judgments, fines and settlement amounts, among others, incurred by such person in any action or proceeding arising out of such person’s services as a director or executive officer in any capacity with respect to any employee benefit plan or as a director, partner, trustee or agent of another entity at our request. We believe that these provisions in our amended and restated certificate of incorporation and amended and restated bylaws and indemnification agreements are necessary to attract and retain qualified persons as directors and executive officers.
The above description of the indemnification provisions of our amended and restated certificate of incorporation, our amended and restated bylaws and our indemnification agreements is not complete and is qualified in its entirety by reference to these documents.
| 81 |
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
Director Compensation
We use a combination of cash and stock-based incentive compensation to attract and retain qualified candidates to serve on our board of directors. In setting director compensation, the board of directors and the compensation committee consider the significant amount of time that directors expend in fulfilling their duties to the Company as well as the skill-level required by the Company of members of the board of directors. Stephen From, our President and CEO, receives no compensation for his service as a director.
Each member of our board of directors who is not our employee is entitled to receive the following cash compensation for board services, as applicable:
| · | $35,000 per year for service as a board of directors member; |
| · | $62,500 per year for service as chairman of the board of directors; |
| · | $15,000 per year for service as chairman of the audit committee; |
| · | $10,000 per year for service as chairman of the compensation committee; |
| · | $7,000 per year for service as chairman of the nominating and corporate governance committee; |
| · | $7,500 per year for service as non-chairman member of the audit committee; |
| · | $5,000 per year for service as non-chairman member of the compensation committee; and |
| · | $3,500 per year for service as non-chairman member of the nominating and corporate governance committee. |
Each new non-employee member of our board of directors that is elected to our board of directors will receive a grant of non-statutory stock options under the 2014 Plan. Such option will be granted following his or her initial election to the board of directors and will be a non-statutory stock option to purchase shares of Common Stock with an exercise price equal to the fair market value of our Common Stock on the grant date. These initial option grants will vest with respect to one-third (1/3) of the underlying shares on the first anniversary of the applicable grant date and ratably in monthly installments over the following 24 months. For purposes of our director grant program, a non-employee director is a director who is not employed by us and who does not receive compensation from us (excluding the non-employee director compensation described above) or have a business relationship with us that would require disclosure under certain SEC rules.
In addition, on the date of each annual meeting of our stockholders, each non-employee director becomes eligible to receive a non-statutory stock option to purchase 15,000 shares of our Common Stock with an exercise price equal to the fair market value of our Common Stock on the grant date. A non-employee director who receives an initial award will not receive the additional annual award in the same calendar year. Automatic annual grants vest in full on the one-year anniversary of the grant date.
All options granted to the non-employee directors as described above will have a maximum term of ten years.
We also reimburse our non-employee directors for their reasonable out-of-pocket expenses incurred in attending board of directors and committee meetings.
| 82 |
Director Compensation Table
The following table presents the compensation provided by us to the non-employee directors who served during the fiscal year ended December 31, 2017.
| Name(1) | Fees earned or paid in cash ($) |
Option awards ($)(2)(3) |
Total ($) |
|||||||||
| Paul Chaney | $ | 72,500 | 17,400 | $ | 89,900 | |||||||
| Morton Goldberg | $ | 38,500 | 17,400 | $ | 55,900 | |||||||
| Praveen Tyle | $ | 59,750 | 17,400 | $ | 77,150 | |||||||
| Thomas Balland | $ | 35,000 | 17,400 | $ | 52,400 | |||||||
| Thomas E. Hancock | $ | 55,000 | 17,400 | $ | 72,400 | |||||||
| Bernard Malfroy-Camine | $ | 47,000 | 17,400 | $ | 64,400 | |||||||
| (1) | Stephen From, our President and Chief Executive Officer is not included in this table as he is our employee and thus receives no compensation for his service as a director. The compensation received by Mr. From as an employee of the Company is shown in the Summary Compensation Table earlier in this proxy statement. |
| (2) | Based on the aggregate grant date fair value computed awards in accordance with the provisions of FASB ASC 718, “Compensation — Stock Compensation” excluding the impact of estimated forfeitures. Assumptions used in the calculation of this amount are included under “Summary of Significant Accounting Policies — Stock-Based Compensation” in Note 2 to our audited financial statements included in this Annual Report on Form 10-K. |
| (3) | The aggregate number of option awards outstanding at our 2017 fiscal year end and held by the non-employee directors were as follows: 129,636 for Mr. Chaney, 54,240 for Mr. Goldberg, 89,333 for Mr. Tyle, 35,192 for Mr. Balland, 35,486 for Mr. Hancock and 52,673 for Mr. Malfroy-Camine. |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
Security Ownership of Certain Beneficial Owners
The following table sets forth information with respect to the beneficial ownership of our Common Stock as of February 23, 2018, by:
| · | each of our named executive officers; |
| · | each of our directors; |
| · | all of our directors and current executive officers as a group; and |
| · | each person or group of affiliated persons known by us to beneficially own more than 5% of our Common Stock. |
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In general, a person is deemed to be the beneficial owner of (i) any shares of our Common Stock over which such person has sole or shared voting power or investment power, plus (ii) any shares which such person has the right to acquire beneficial ownership of within 60 days of February 23, 2018, whether through the exercise of options, warrants or otherwise.
| 83 |
| Common Stock Beneficially Owned |
||||||||
| Name of Beneficial Owner(1) | Shares | Percent(2) | ||||||
| 5% or Greater Stockholders | ||||||||
| Entities affiliated with Ventech SA(3) 47, avenue de l’Opéra Paris, France 75002 |
2,773,718 | 15.9 | % | |||||
| Armistice Capital Master Fund, Ltd.(4) 510 Madison Avenue, 22nd Floor New York, NY 10022 |
1,284,000 | 7.4 | % | |||||
| Executive Officers and Directors | ||||||||
| Stephen From(5) | 868,649 | 4.9 | % | |||||
| Barbara Wirostko(6) | 322,732 | 1.9 | % | |||||
| Michael Manzo(7) | 152,122 | * | ||||||
| Paul Chaney(8) | 150,584 | * | ||||||
| Morton Goldberg(9) | 45,111 | * | ||||||
| Praveen Tyle(10) | 82,587 | * | ||||||
| Thomas Balland(11) | 798,983 | 4.6 | % | |||||
| Thomas E. Hancock(12) | 32,187 | * | ||||||
| Bernard Malfroy-Camine(13) | 46,796 | * | ||||||
| All current executive officers and directors as a group (total 9 persons)(14) | 2,499,751 | 13.6 | % | |||||
| * | Represents beneficial ownership of less than one percent (1%) of our outstanding Common Stock. |
| (1) | Unless otherwise indicated, the address of each beneficial owner listed below is c/o EyeGate Pharmaceuticals, Inc., 271 Waverley Oaks Road, Suite 108, Waltham, MA 02452. |
| (2) | Based on 17,257,255 shares of Common Stock outstanding on February 23, 2018, together with the applicable options for each stockholder that become exercisable within 60 days. |
| (3) | This information is based solely upon an amendment to Schedule 13D filed jointly by FCPR Ventech A, FCPR Ventech B, FCRP Ventech Coinvest and FCPR Ventech Capital II with the Securities and Exchange Commission on June 16, 2017 reporting beneficial ownership as of June 14, 2017. Consists of: |
| (a) | 512,379 shares and 62,384 warrants held by FCPR Ventech A; (b) 593,205 shares and 15,668 warrants held by FCPR Ventech B; |
| (c) | 961 shares held by FCPR Ventech Coinvest; and |
| (d) | 1,431,814 shares and 157,307 warrants held by FCPR Ventech Capital II. |
Alain Caffi and Jean Bourcereau, as directors of Ventech SA, have voting and investment power with respect to the shares held by all of the foregoing entities.
| (4) | This information is based solely upon an amendment to Schedule 13G filed jointly by Armistice Capital, LLC, Armistice Capital Master Fund Ltd. and Steven Boyd with Securities and Exchange Commission on February 14, 2018 reporting beneficial ownership as of February 14, 2018. Consists of 1,284,000 shares owned by Armistice Capital Master Fund Ltd. Armistice Capital, LLC and Steven Boyd have voting and investment power with respect to such shares. |
| (5) | Consists of 284,820 shares held and 523,829 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018 and 60,000 shares issuable pursuant to warrants exercisable within 60 days of February 23, 2018. |
| (6) | Consists of 291,097 shares held (including 50,700 shares held by Dr. Wirostko’s husband) and 31,635 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. |
| (7) | Consists of 19,546 shares held and 132,576 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. |
| (8) | Consists of 41,229 shares held and 109,355 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. |
| (9) | Consists of 9,441 shares held and 35,670 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. |
| 84 |
| (10) | Consists of 11,893 shares held and 70,694 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. |
| (11) | Consists of 770,065 shares beneficially owned as a director of IPSA and its affiliates (including 89,172 shares issuable upon exercise of warrants exercisable within 60 days of February 23, 2018 beneficially owned as a director of IPSA), 10,422 shares held individually and 18,496 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. (12) Consists of 13,487 shares held and 18,700 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. |
| (13) | Consists of 11,525 shares held and 35,271 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. |
| (14) | Consists of 1,374,353 shares held, 149,172 shares issuable upon exercise of warrants exercisable within 60 days of February 23, 2018 and 976,226 shares issuable pursuant to stock options exercisable within 60 days of February 23, 2018. |
Equity Compensation Plan Information
The following table sets forth information as of December 31, 2017 concerning the number of shares of Common Stock issuable under our existing equity compensation plans.
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Restricted Stock Units, Warrants and Rights |
Weighted Average Exercise Price of Outstanding Options, Warrants, and Rights |
Number of Securities Remaining Available For Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) |
|||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders(1) | 2,488,714 | $ | 2.49 | 116,411 | ||||||||
| Equity compensation plans not approved by security holders | — | — | — | |||||||||
| Total | 2,488,714 | $ | 2.49 | 116,411 | ||||||||
| (1) | Consists of our 2014 Plan and our 2005 Plan. |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence. |
The following is a description of transactions since January 1, 2017 to which we have been a party, in which the amount involved exceeded or will exceed the average of 1% of our total assets as of December 31, 2016 and December 31, 2017, and in which any of our directors, executive officers or beneficial owners of more than 5% of our Common Stock, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest, other than compensation, termination and change-in-control arrangements, which are described under “Executive Compensation.” We also describe below certain other transactions with our directors, executive officers and stockholders.
All of the transactions set forth below were approved by a majority of our board of directors, including a majority of the independent and disinterested members of our board of directors. We believe that we have executed all of the transactions set forth below on terms no less favorable to us than we could have obtained from unaffiliated third parties. It is our intention to ensure that all future transactions between us and our officers, directors and principal stockholders and their affiliates are approved by the audit committee and a majority of the members of our board of directors, including a majority of the independent and disinterested members of our board of directors, and are on terms no less favorable to us than those that we could obtain from unaffiliated third parties.
Indemnification Agreements
We have entered into separate indemnification agreements with our directors and executive officers, in addition to indemnification provided for in our restated certificate of incorporation and amended and restated bylaws. These agreements, among other things, provide for indemnification of our directors and executive officers for certain expenses, judgments, fines and settlement amounts, among others, incurred by such person in any action or proceeding arising out of such person’s services as a director or executive officer in any capacity with respect to any employee benefit plan or as a director, partner, trustee or agent of another entity at our request. We believe that these provisions in our restated certificate of incorporation and amended and restated bylaws and indemnification agreements are necessary to attract and retain qualified persons as directors and executive officers.
Director Independence
The board of directors has determined that the directors listed in Part III, Item 10 of this Annual Report on Form 10-K are “independent” as such term is currently defined by applicable NASDAQ rules, except for Mr. From who is also an executive officer of the Company.
The Board of Directors has determined that all members of the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee of the Board of Directors are “independent” as such term is currently defined by NASDAQ rules. Additionally, the Board has Directors has determined that all members of the Audit Committee of the Board of Directors meet the criteria for independence set forth under the rules of the Securities and Exchange Commission.
| 85 |
| Item 14. | Principal Accounting Fees and Services. |
Fees for professional services provided by EisnerAmper LLP, our independent registered public accounting firm, during the fiscal years ended December 31, 2016 and December 31, 2017, in each of the following categories is as set forth in the table below.
| 2016 | 2017 | |||||||
| Audit Fees(1) | $ | 171,621 | $ | 272,200 | ||||
| Audit-Related Fees(2) | $ | 18,000 | $ | — | ||||
| Tax Fees(3) | $ | — | $ | — | ||||
| All Other Fees(4) | $ | — | $ | — | ||||
| Total Fees | $ | 189,621 | $ | 272,200 | ||||
| (1) | Audit Fees include fees for services rendered for the audit of our annual consolidated financial statements, the review of financial statements included in our quarterly reports on Form 10-Q, assistance with and review of documents filed with the SEC and consents and other services normally provided in connection with statutory and regulatory filings or engagements. |
| (2) | Audit-Related Fees would principally include fees incurred for due diligence in connection with potential transactions and accounting consultations. For 2016, such fees were incurred in connection with the Jade Acquisition. |
| (3) | Tax Fees would include fees for services rendered for tax compliance, tax advice, and tax planning. There were no tax fees incurred with EisnerAmper LLP in 2017 and 2016. |
| (4) | All Other Fees would include fees for all other services rendered to us that do not constitute Audit Fees, Audit-Related Fees, or Tax Fees. There were no other fees incurred with EisnerAmper LLP in 2017 and 2016. |
All of the services performed in the years ended December 31, 2016 and December 31, 2017 were pre-approved by the audit committee. It is the audit committee’s policy to pre-approve all audit and permitted non-audit services to be provided to us by the independent registered public accounting firm. The audit committee’s authority to pre-approve non-audit services may be delegated to one or more members of the audit committee, who shall present all decisions to pre-approve an activity to the full audit committee at its first meeting following such decision. In addition, the audit committee has considered whether the provision of the non-audit services above is compatible with maintaining the independent registered public accounting firm’s independence.
| 86 |
| Item 15. | Exhibits, Financial Statement Schedules. |
| (a) | Documents Filed. The following documents are filed as part of this Annual Report on Form 10-K: |
| (1) | Financial Statements. The Consolidated Financial Statements of EyeGate Pharmaceuticals, Inc. and its subsidiaries filed under this Item 15: |
| (2) | Financial Statement Schedules: None. Financial statement schedules have been omitted since the required information is included in our Consolidated Financial Statements contained elsewhere in this Annual Report on Form 10-K. |
| (3) | Exhibits. The exhibits listed in the accompanying Exhibit Index are filed as a part of this Annual Report on Form 10-K. |
| (b) | Exhibits: The exhibits listed in the accompanying Exhibit Index are filed as a part of this Annual Report on Form 10-K. |
| (c) | Separate Financial Statements and Schedules: None. Financial statement schedules have been omitted since the required information is included in our Consolidated Financial Statements contained elsewhere in this Annual Report on Form 10-K. |
| 87 |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
EYEGATE PHARMACEUTICALS, INC.
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
EyeGate Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of EyeGate Pharmaceuticals, Inc. (the “Company”) as of December 31, 2017 and 2016, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the years then ended and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2017 and 2016, and the consolidated results of their operations and their cash flows for each of the years then ended in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred operating losses from operations and negative cash flows that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ EisnerAmper LLP |
We have served as the Company’s auditor since 2014.
EISNERAMPER LLP
New York, New York
March 2, 2018
| F-2 |
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash and Cash Equivalents | $ | 7,806,029 | $ | 3,635,224 | ||||
| License and Grant Fees Receivable | - | 37,349 | ||||||
| Prepaid Expenses and Other Current Assets | 629,591 | 464,981 | ||||||
| Current Portion of Refundable Tax Credit Receivable | 23,685 | 16,484 | ||||||
| Total Current Assets | 8,459,305 | 4,154,038 | ||||||
| Property and Equipment, Net | 55,751 | 38,040 | ||||||
| Restricted Cash | 45,000 | 45,000 | ||||||
| Goodwill and In-Process R&D | 5,438,210 | 5,438,210 | ||||||
| Other Assets | 307,126 | 55,314 | ||||||
| Total Assets | $ | 14,305,392 | $ | 9,730,602 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current Liabilities: | ||||||||
| Accounts Payable | $ | 706,089 | $ | 1,412,128 | ||||
| Accrued Expenses | 1,813,847 | 1,670,930 | ||||||
| Deferred Revenue | 12,313,600 | 4,225,000 | ||||||
| Total Current Liabilities | 14,833,536 | 7,308,058 | ||||||
| Non-Current Liabilities: | ||||||||
| Contingent Consideration | 1,210,000 | 1,210,000 | ||||||
| Deferred Tax Liability | 183,923 | 1,525,896 | ||||||
| Long-Term Portion of Capital Lease Obligation | 4,855 | 16,069 | ||||||
| Total Non-Current Liabilities | 1,398,778 | 2,751,965 | ||||||
| Total Liabilities | 16,232,314 | 10,060,023 | ||||||
| Commitments and Contingencies (Note 11) | ||||||||
| Stockholders’ Deficit: | ||||||||
| Preferred Stock, $0.01 Par Value: 9,995,828 shares authorized; 3,750 designated Series A, 0 shares issued and outstanding at December 31, 2017 and December 31, 2016; 10,000 designated Series B, 600 and 0 issued and outstanding at December 31, 2017 and December 31, 2016, respectively | 6 | - | ||||||
| Common Stock, $0.01 Par Value: 100,000,000 shares authorized; 17,257,255 shares issued and outstanding at December 31, 2017 and 10,130,883 shares issued and outstanding at December 31, 2016 | 172,573 | 101,309 | ||||||
| Additional Paid-In Capital | 89,589,681 | 78,106,645 | ||||||
| Accumulated Deficit | (91,816,655 | ) | (78,598,738 | ) | ||||
| Stockholders’ Notes Receivable | - | (58,824 | ) | |||||
| Accumulated Other Comprehensive Income | 127,473 | 120,187 | ||||||
| Total Stockholders’ Deficit | (1,926,922 | ) | (329,421 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 14,305,392 | $ | 9,730,602 | ||||
See Accompanying Notes to the Consolidated Financial Statements.
| F-3 |
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Collaboration Revenue | $ | 407,518 | $ | 669,259 | ||||
| Operating Expenses: | ||||||||
| Research and Development | 10,330,349 | 8,422,542 | ||||||
| General and Administrative | 4,636,408 | 5,593,563 | ||||||
| Total Operating Expenses | 14,966,757 | 14,016,105 | ||||||
| Other (Expense) Income, Net: | ||||||||
| Interest Income | 564 | 3,684 | ||||||
| Interest Expense | (1,215 | ) | (275 | ) | ||||
| Total Other (Expense) Income, Net | (651 | ) | 3,409 | |||||
| Loss Before Income Tax Benefit | (14,559,890 | ) | (13,343,437 | ) | ||||
| Income Tax Benefit | 1,341,973 | - | ||||||
| Net Loss | $ | (13,217,917 | ) | $ | (13,343,437 | ) | ||
| Net Loss per Common Share - Basic and Diluted | $ | (0.93 | ) | $ | (1.51 | ) | ||
| Weighted Average Shares Outstanding - Basic and Diluted | 14,260,103 | 8,833,898 | ||||||
| Other Comprehensive Loss: | ||||||||
| Foreign Currency Translation Adjustments | 7,286 | 5,399 | ||||||
| Comprehensive Loss | $ | (13,210,631 | ) | $ | (13,338,038 | ) | ||
See Accompanying Notes to the Consolidated Financial Statements.
| F-4 |
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
| Series A | Series B | |||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||
| Balance at December 31, 2015 | - | $ | - | - | $ | - | ||||||||||
| Stock-Based Compensation |
||||||||||||||||
| Shares Issued to Jade Therapeutics, Inc. Stockholders at Acquisition | ||||||||||||||||
| Issuance of Holdback Shares from the Jade Acquisition | ||||||||||||||||
| Forfeiture of Holdback Shares from the Jade Acquisition | ||||||||||||||||
| Issuance of Common Stock in Offering, Net of Offering Costs of $85,260 | ||||||||||||||||
| Issuance of Series A Preferred Stock, Net of Offering Costs of $238,554 | 2,777 | 28 | ||||||||||||||
| Conversion of Series A Preferred | (2,777 | ) | (28 | ) | ||||||||||||
| Exercise of Common Stock Options | ||||||||||||||||
| Foreign Currency Translation Adjustment | ||||||||||||||||
| Net Loss | ||||||||||||||||
| Balance at December 31, 2016 | - | $ | - | - | $ | - | ||||||||||
| Additional | Stockholders’ | Accumulated Other |
Total Stockholders’ |
|||||||||||||||||||||||||||||||||
| Common Stock | Common Stock Held for Issue | Paid In | Notes | Comprehensive | Accumulated | (Deficit) | ||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Receivable | Income | Deficit | Equity | ||||||||||||||||||||||||||||
| Balance at December 31, 2015 | 7,657,287 | $ | 76,573 | - | $ | - | $ | 71,209,530 | $ | (58,824 | ) | $ | 114,788 | $ | (65,255,301 | ) | $ | 6,086,766 | ||||||||||||||||||
| Stock-Based Compensation | 510,995 | 510,995 | ||||||||||||||||||||||||||||||||||
| Shares Issued to Jade Therapeutics, Inc. Stockholders at Acquisition | 689,157 | 6,891 | 76,571 | 291,536 | 2,611,339 | 2,909,766 | ||||||||||||||||||||||||||||||
| Issuance of Holdback Shares from the Jade Acquisition | 22,674 | 227 | (22,674 | ) | (86,329 | ) | 86,102 | - | ||||||||||||||||||||||||||||
| Forfeiture of Holdback Shares from the Jade Acquisition | (53,897 | ) | (205,207 | ) | 205,207 | - | ||||||||||||||||||||||||||||||
| Issuance of Common Stock in Offering, Net of Offering Costs of $85,260 | 441,000 | 4,410 | 664,027 | 668,437 | ||||||||||||||||||||||||||||||||
| Issuance of Series A Preferred Stock Net of Offering Costs of $238,554 | 2,776,419 | 2,776,447 | ||||||||||||||||||||||||||||||||||
| Conversion of Series A Preferred Stock | 1,234,000 | 12,340 | (12,312 | ) | - | |||||||||||||||||||||||||||||||
| Exercise of Common Stock Options | 86,765 | 868 | 55,338 | 56,206 | ||||||||||||||||||||||||||||||||
| Foreign Currency Translation Adjustment | 5,399 | 5,399 | ||||||||||||||||||||||||||||||||||
| Net Loss | (13,343,437 | ) | (13,343,437 | ) | ||||||||||||||||||||||||||||||||
| Balance at December 31, 2016 | 10,130,883 | $ | 101,309 | - | $ | - | $ | 78,106,645 | $ | (58,824 | ) | $ | 120,187 | $ | (78,598,738 | ) | $ | (329,421 | ) | |||||||||||||||||
See Accompanying Notes to the Consolidated Financial Statements.
| F-5 |
EYEGATE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
| Series A | Series B | |||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||
| Balance at December 31, 2016 | - | $ | - | - | $ | - | ||||||||||
| Stock-Based Compensation |
||||||||||||||||
| Cancellation of Stockholder Note Receivable | ||||||||||||||||
| Issuance of Common Stock in Offerings, Net of Offering Costs of $1,086,736 | ||||||||||||||||
| Issuance of Series B Preferred Stock, Net of Offering Costs of $246,333 | 1,995 | 20 | ||||||||||||||
| Conversion of Series B Preferred Stock | (1,395 | ) | (14 | ) | ||||||||||||
| Exercise of Common Stock Options | ||||||||||||||||
| Issuance of Restricted Stock, Net of Cancellations | ||||||||||||||||
| Issuance of Common Stock from Employee Stock Purchase Plan | ||||||||||||||||
| Foreign Currency Translation Adjustment | ||||||||||||||||
| Net Loss | ||||||||||||||||
| Balance at December 31, 2017 | - | $ | - | 600 | $ | 6 | ||||||||||
| Additional | Stockholders’ | Accumulated Other |
Total Stockholders’ |
|||||||||||||||||||||||||
| Common Stock | Paid In | Notes | Comprehensive | Accumulated | (Deficit) | |||||||||||||||||||||||
| Shares | Amount | Capital | Receivable | Income | Deficit | Equity | ||||||||||||||||||||||
| Balance at December 31, 2016 | 10,130,883 | $ | 101,309 | $ | 78,106,645 | $ | (58,824 | ) | $ | 120,187 | $ | (78,598,738 | ) | $ | (329,421 | ) | ||||||||||||
| Stock-Based Compensation | 870,308 | 870,308 | ||||||||||||||||||||||||||
| Cancellation of Stockholder Note Receivable | 58,824 | 58,824 | ||||||||||||||||||||||||||
| Issuance of Common Stock in Offerings, Net of Offering Costs of $1,086,736 | 5,978,817 | 59,788 | 8,551,895 | 8,611,683 | ||||||||||||||||||||||||
| Issuance of Series B Preferred Stock, Net of Offering Costs of $246,333 | 1,977,480 | 1,977,500 | ||||||||||||||||||||||||||
| Conversion of Series B Preferred Stock | 930,000 | 9,300 | (9,286 | ) | - | |||||||||||||||||||||||
| Exercise of Common Stock Options | 61,078 | 611 | 40,107 | 40,718 | ||||||||||||||||||||||||
| Issuance of Restricted Stock, Net of Cancellations | 103,000 | 1,030 | (1,030 | ) | - | |||||||||||||||||||||||
| Issuance of Common Stock from Employee Stock Purchase Plan | 53,477 | 535 | 53,562 | 54,097 | ||||||||||||||||||||||||
| Foreign Currency Translation Adjustment | 7,286 | 7,286 | ||||||||||||||||||||||||||
| Net Loss | (13,217,917 | ) | (13,217,917 | ) | ||||||||||||||||||||||||
| Balance at December 31, 2017 | 17,257,255 | $ | 172,573 | $ | 89,589,681 | $ | - | $ | 127,473 | $ | (91,816,655 | ) | $ | (1,926,922 | ) | |||||||||||||
See Accompanying Notes to the Consolidated Financial Statements.
| F-6 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Operating Activities | ||||||||
| Net Loss | $ | (13,217,917 | ) | $ | (13,343,437 | ) | ||
| Adjustments to Reconcile Net Loss to Net Cash Used in Operating Activities: | ||||||||
| Depreciation and Amortization | 19,288 | 5,185 | ||||||
| Stock-Based Compensation | 870,308 | 510,995 | ||||||
| Loss on Cancellation of Stockholder Note Receivable | 91,054 | - | ||||||
| Deferred Taxes | (1,341,973 | ) | - | |||||
| Changes in Operating Assets and Liabilities: | ||||||||
| License and Grant Receivable | 37,349 | 3,233,636 | ||||||
| Prepaid Expenses and Other Current Assets | (447,570 | ) | 26,287 | |||||
| Refundable Tax Credit Receivable | (4,907 | ) | 7,703 | |||||
| Other Assets | (1,082 | ) | (16,727 | ) | ||||
| Accounts Payable | (706,039 | ) | 712,795 | |||||
| Deferred Revenue | 8,088,600 | - | ||||||
| Accrued Expenses | 144,348 | 450,383 | ||||||
| Net Cash Used in Operating Activities | (6,468,541 | ) | (8,413,180 | ) | ||||
| Investing Activities: | ||||||||
| Equipment Purchased Under Capital Lease | - | (11,000 | ) | |||||
| Purchase of Property and Equipment | (36,999 | ) | - | |||||
| Acquisition of Jade (Net of Cash Acquired) | - | 185,746 | ||||||
| Net Cash (Used in) Provided by Investing Activities | (36,999 | ) | 174,746 | |||||
| Financing Activities: | ||||||||
| Exercise of Common Stock Options | 40,718 | 56,206 | ||||||
| Proceeds from Employee Stock Purchase Plan | 54,097 | |||||||
| Proceeds from Stock Offerings | 11,922,252 | 3,768,698 | ||||||
| Stock Issuance Costs | (1,333,069 | ) | (323,814 | ) | ||||
| Equipment Financing Payments | (12,645 | ) | (2,863 | ) | ||||
| Net Cash Provided by Financing Activities | 10,671,353 | 3,498,227 | ||||||
| Effect of Exchange Rate Changes on Cash | 4,992 | 6,298 | ||||||
| Net Increase (Decrease) in Cash | 4,170,805 | (4,733,909 | ) | |||||
| Cash, Including Restricted Cash, Beginning of Year | 3,680,224 | 8,414,133 | ||||||
| Cash, Including Restricted Cash, End of Year | $ | 7,851,029 | $ | 3,680,224 | ||||
See Accompanying Notes to the Consolidated Financial Statements.
| F-7 |
EYEGATE PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Supplemental Disclosure of Noncash Investing and Financing Activities: | ||||||||
| Conversion of Preferred Stock into Common Stock | $ | 9,300 | $ | 2,776,419 | ||||
| Issuance of Common Stock to Acquire Jade Therapeutics, Inc. | $ | - | $ | 2,909,766 | ||||
| Contingent Liability in Connection with Jade Acquisition | $ | - | $ | 1,210,000 | ||||
| Property and Equipment Acquired Under Capital Lease | $ | - | $ | 31,576 | ||||
See Accompanying Notes to the Consolidated Financial Statements.
| F-8 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
1. Organization, Business
EyeGate Pharmaceuticals, Inc. (“EyeGate”or the “Company”) a Delaware corporation, began operations in December 2004 and is a clinical-stage specialty pharmaceutical company that is focused on developing and commercializing products for treating diseases and disorders of the eye. EyeGate’s first product in clinical trials incorporates a reformulated topically active corticosteroid, dexamethasone phosphate, EGP-437, that is delivered into the ocular tissues though our proprietary iontophoresis drug delivery system, the EyeGate® II Delivery System. The Company is developing the EyeGate® II Delivery System and EGP-437 combination product (together, the “EGP-437 Product”) for the treatment of various inflammatory conditions of the eye, including anterior uveitis, a debilitating form of intraocular inflammation of the anterior portion of the uvea, such as the iris and/or ciliary body, post-cataract surgery inflammation and pain, and macular edema, an abnormal thickening of the macula associated with the accumulation of excess fluids in the retina. Effective March 7, 2016, the Company acquired all of the capital stock of Jade Therapeutics, Inc. (“Jade”), a privately-held company developing locally-administered, polymer-based products designed to treat poorly-served ophthalmic indications (the “Jade Acquisition”). EyeGate and Jade are an integrated line of business developing ophthalmic solutions for a variety of ocular diseases and disorders.
On June 30, 2016, the Company completed a subsequent registered direct offering of 441,000 shares of Common Stock and 2,776.5 shares of Series A Preferred Stock (convertible into 1,234,000 shares of Common Stock), along with a concurrent private placement of warrants to purchase Common Stock. The total net proceeds to the Company from this subsequent offering, after deducting the placement agent fees and offering expenses, were approximately $3.4 million. The warrants are initially exercisable on December 30, 2016, and expire on December 30, 2021. On February 21, 2017, the Company authorized the restart of sales under the At The Market Offering Agreement between the Company and H.C. Wainwright & Co., LLC (the “ATM Agreement”) and subsequently sold 642,150 shares of Common Stock during the first quarter of 2017. No shares of Common Stock were sold pursuant to the ATM Agreement during the second, third or fourth quarters of 2017. The total net proceeds to the Company from this offering, after deducting the placement agent fees and offering expenses, were approximately $1.8 million. On June 14, 2017, the Company completed a public offering of 5,336,667 shares of Common Stock and 1,995 shares of Series B Preferred Stock (convertible into 1,330,000 shares of Common Stock), along with warrants to purchase 6,666,667 shares of Common Stock. The total net proceeds to the Company from the offering, after deducting the placement agent fees and offering expenses, were approximately $8.8 million. The warrants became exercisable upon issuance, and expire on June 14, 2022. See Note 6, “Capital Stock”.
As of December 31, 2017, there were 17,257,255 shares of Common Stock outstanding, $0.01 par value, no shares of Series A Preferred Stock outstanding, $0.01 par value, and 600 shares of Series B Preferred Stock outstanding, $0.01 par value.
Effective July 31, 2015, the Company’s Common Stock began trading on the Nasdaq Capital Market under the symbol “EYEG”.
Since its inception, EyeGate has devoted substantially all of its efforts to business planning, research and development, and raising capital.
The accompanying Consolidated Financial Statements have been prepared assuming that EyeGate will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. At December 31, 2017, EyeGate had Cash and Cash Equivalents of $7,806,029, and an Accumulated Deficit of $91,816,655. EyeGate has incurred losses and negative cash flows since inception, and future losses are anticipated. The Company anticipates having sufficient cash to fund planned operations for approximately seven months, however, the acceleration or reduction of cash outflows by Company management can significantly impact the timing for raising additional capital to complete development of its products. To continue development, EyeGate will need to raise additional capital through equity financing, license agreements, and/or additional U.S. government grants. Although the Company successfully completed its IPO, a follow-on offering, a registered direct offering, a public offering, and sales under the ATM Agreement, additional capital may not be available on terms favorable to EyeGate, if at all. On May 6, 2016, the SEC declared effective EyeGate’s registration statement on Form S-3, registering a total of $100,000,000 of its securities for sale to the public from time to time in what is known as a “shelf offering”. The Company does not know if any future offerings pursuant to its shelf registration statement will succeed. Accordingly, no assurances can be given that Company management will succeed in these endeavors. The Company’s recurring losses from operations have caused management to determine there is substantial doubt about the Company’s ability to continue as a going concern. The Consolidated Financial Statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities or any other adjustments that might be necessary should the Company be unable to continue as a going concern.
| F-9 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
2. Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The accompanying Consolidated Financial Statements include the accounts of the Company and its subsidiaries, EyeGate Pharma S.A.S. and Jade (since the date of the Jade Acquisition), collectively referred to as “the Company”. All inter-company balances and transactions have been eliminated in consolidation. These Consolidated Financial Statements have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”).
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make significant estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities, at the date of the financial statements, and the reported amounts of expenses during the reporting periods. The Company makes significant estimates and assumptions in recording the accruals for our clinical trial and research activities, establishing the useful lives of intangible assets and property and equipment, and conducting impairment reviews of long-lived assets. The Company bases its estimates on historical experience and various other assumptions that it believes to be reasonable under the circumstances. Although the Company monitors and regularly assesses these estimates, actual results could differ significantly from these estimates. The Company records changes in estimates in the period that it becomes aware of the change.
Foreign Currency Translation
Operations of EyeGate Pharma S.A.S. are conducted in euros which represent its functional currency. Balance sheet accounts of such subsidiary were translated into U.S. dollars at the exchange rate in effect at the balance sheet date and income statement accounts were translated to the average rate of exchange prevailing during the period. Translation adjustments resulting from this process, are included in accumulated other comprehensive loss on the Consolidated Balance Sheets.
Cash and Cash Equivalents and Restricted Cash
The Company considers all highly liquid investments purchased with maturity of 90 days or less when acquired that are not restricted as to withdrawal, to be the equivalent of cash for the purpose of balance sheet and statement of cash flows presentation. Cash equivalents, which were nominal in amount, consisted of money market accounts that are readily convertible to cash. As of December 31, 2017 and 2016, the Company has classified $45,000 and $45,000 as restricted cash, respectively.
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Depreciation is provided for on a straight-line basis over the estimated useful life of 2 to 5 years for all assets. Maintenance and repair costs are expensed as incurred. The Company reviews its property and equipment whenever events or changes in circumstances indicate that the carrying value of certain assets might not be recoverable, and recognizes an impairment loss when it is probable that the estimated cash flows are less than the carrying value of the asset.
| F-10 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
2. Summary of Significant Accounting Policies - (continued)
Impairment of Long-Lived Assets
The Company evaluates potential impairment of long-lived assets and long-lived assets to be disposed of and considers whether long-lived assets held for use have been impaired whenever events or changes in circumstances indicate that the related carrying amount may not be recoverable, or that the period of their recovery may have changed. Management makes significant estimates and assumptions regarding future sales, cost trends, productivity and market maturity in order to test for impairment. Management reports those long-lived assets to be disposed of and assets held for sale at the lower of carrying amount or fair value less cost to sell. Based on current facts, estimates and assumptions, management believes that no assets are impaired at December 31, 2017. There is no assurance that management’s estimates and assumptions will not change in future periods.
Research and Development Expenses
The Company expenses research and development (“R&D”) expenditures as incurred. R&D expenses are comprised of costs incurred in performing R&D activities, including salaries, benefits, facilities, research-related overhead, sponsored research costs, contracted services, license fees, expenses related to generating, filing, and maintaining intellectual property and other external costs. Because the Company believes that, under its current process for developing its products, the viability of the products is essentially concurrent with the establishment of technological feasibility, no costs have been capitalized to date.
Goodwill
Goodwill is the excess of the acquisition cost of a business over the fair value of the identifiable net assets acquired. Goodwill at December 31, 2017 was $1,525,896, which solely consists of the goodwill acquired in the acquisition of Jade.
Goodwill is not amortized and is tested for impairment on an annual basis in the fourth quarter of each fiscal year and whenever events or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount.
The Company performed qualitative impairment evaluations on its goodwill as of December 31, 2017 and determined that there were no indications that goodwill was impaired.
In-Process Research and Development
The Company records in-process R&D projects acquired as asset acquisitions that have not reached technological feasibility and which have no alternative future use. For in-process R&D projects acquired in business combinations, the Company capitalizes the in-process R&D project and periodically evaluates this asset for impairment until the R&D process has been completed. Once the R&D process is complete, the Company amortizes the R&D asset over its remaining useful life. At December 31, 2017, the Company has recorded $3,912,314 as in-process R&D in connection with the Jade Acquisition as part of goodwill and in-process R&D.
Accrued Clinical Expenses
As part of our process of preparing the Consolidated Financial Statements, the Company is required to estimate its accrued expenses. This process includes reviewing open contracts and purchase orders, communicating with its applicable personnel to identify services that have been performed on its behalf and estimating the level of service performed and the associated costs incurred for the service when the Company has not yet been invoiced or otherwise notified of actual costs. The majority of the Company’s service providers invoice monthly in arrears for services performed. The Company makes estimates of its accrued expenses as of each balance sheet date in the financial statements based on facts and circumstances known at the time. The Company periodically confirms the accuracy of these estimates with the service providers and makes adjustments if necessary.
Business Segment and Geographical Information
The Company identifies operating segments as components of the enterprise for which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision making group, in making decisions on how to allocate resources and assess performance. The Company views its operations and manages its business as fully integrated and operating in one business segment (research and development), and the Company operates in one geographic segment.
| F-11 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
2. Summary of Significant Accounting Policies - (continued)
Income Taxes
The Company will record a deferred income tax asset and liability for the expected future income tax consequences of events that have been recognized in the Company’s Consolidated Financial Statements and income tax returns. The Company will record a deferred income tax asset and liability based on differences between the financial statement carrying, or “book”, amounts of assets and liabilities, and the tax bases of the assets and liabilities using the enacted income tax regulations in effect in the years in which the differences are expected to reverse. A valuation allowance against deferred income tax asset will be recorded if, based on the weight of available evidence, it is more likely than not that some or all of the deferred income tax assets will not be realized. As of December 31, 2017 and 2016, all of the Company’s net deferred income tax assets were subject to a full valuation allowance. As of December 31, 2017 and 2016, the Company has a net deferred tax liability of $183,923 and $1,525,896, respectively.
On December 22, 2017, the passage of legislation commonly referred to as the Tax Cuts and Jobs Act (“TCJA”) was enacted and significantly revised the U.S. income tax law. The TCJA includes changes, which reduce the corporate income tax rate from 34% to 21% for years beginning after December 31, 2017 and transitions the U.S. towards a territorial tax system where U.S. shareholders of certain foreign subsidiaries would be subject to a one-time transition tax on unrepatriated accumulated foreign earnings. On December 22, 2017, Staff Accounting Bulletin No. 118 (“SAB 118”) was issued and allows a company to recognize provisional amounts when it does not have the necessary information available, prepared or analyzed, including computations, in reasonable detail to complete its accounting for the change in tax law. SAB 118 provides for a measurement of up to one year from the date of enactment.
The Company recognizes the impact of an uncertain income tax position in the financial statements if it believes that the position is more likely than not to be sustained by the relevant taxing authority. As of December 31, 2017, the Company had no unrecognized uncertain income tax positions.
Refundable Tax Credits for Research and Development
EyeGate is entitled to receive refundable tax credits associated with its research and development expenses in France. These tax credits can be realized, upon request of the Company, in the form of a cash payment or credits against tax liabilities. The Company records the refundable tax credit as income in the year in which the research and development expenses are incurred.
Concentration of Credit Risk and Off-Balance-Sheet Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents. The Company invests cash in accredited financial institutions and cash equivalents in widely held money market funds. Consequently, such funds are subject to minimal credit risk.
The Company has no significant off-balance-sheet risk such as foreign exchange contracts, option contracts, or other foreign hedging arrangements.
| F-12 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
2. Summary of Significant Accounting Policies - (continued)
Comprehensive Loss
Comprehensive loss is defined as the change in stockholders’ equity (deficit) during a period from transactions, and other events and circumstances from non-owner sources. The foreign currency translation adjustments (see above) are the Company’s only component of other comprehensive loss.
Stock-Based Compensation
Stock-based compensation represents the cost related to stock-based awards granted to employees and others. The Company measures stock-based compensation cost to employees at grant date, based on the estimated fair value of the award, and recognizes the cost as expense on a straight-line basis over the employee requisite service period. The Company estimates the fair value of stock options using the Black-Scholes valuation model. The Company recognizes compensation expense for non-employee stock option grants at the fair value of the goods or services received or the equity instruments issued, whichever is more reliably measurable. The Company recorded compensation expense for non-employee awards with graded vesting using the accelerated expense attribution method. In applying the Black-Sholes valuation model, prior to July 1, 2016 the Company estimated the volatility factor in the valuation calculation by using the historic stock volatility of a group of peer public companies. Effective July 1, 2016, the Company determined that the prior methodology for measuring the volatility of its Common Stock was no longer the best estimate of volatility, and the Company will instead measure volatility using its own Common Stock volatility. The Company believes that the public market for its Common Stock is the best measure to use as an input in the option pricing model. All future grants of stock options will use the Company’s historic Common Stock volatility. The Company adopted ASU No. 2016-09, Compensation - Stock Compensation, as of January 1, 2017. The Company’s policy is to record forfeitures as they occur.
Net Loss per Share – Basic and Diluted
The computation of Net Loss per Common Share – Basic and Diluted, is based on the weighted-average number of shares outstanding Common Stock.
In computing diluted loss per share, no effect has been given to the shares of Common Stock issuable upon the conversion or exercise of the following dilutive securities as the Company’s net loss would make the effect anti-dilutive.
| December 31, 2017 |
December 31, 2016 |
|||||||
| Common Stock Warrants | 9,455,961 | 2,852,736 | ||||||
| Employee Stock Options | 1,893,003 | 1,509,711 | ||||||
| Preferred Stock | 400,000 | - | ||||||
| Total Shares of Common Stock Issuable | 11,748,964 | 4,362,447 | ||||||
| F-13 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
2. Summary of Significant Accounting Policies - (continued)
Related-Party Transactions
The Company has entered into certain related-party transactions, making payments for services to two vendors, eleven consultants and two public universities, all of whom also are stockholders of the Company. These transactions generally are ones that involve a stockholder or option holder of the Company to whom we also make payments during the year, typically as a consultant or a service provider. The amounts recorded or paid are not material to the accompanying Consolidated Financial Statements.
Fair Value of Financial Instruments
The carrying amounts of Accounts Receivable and Accounts Payable approximate their fair values due to the short-term nature of these items. As December 31, 2017 and December 31, 2016, the fair value of the Company’s money market funds and contingent consideration was $750,965 and $1,210,000, and $1,500,882 and $1,210,000, respectively.
At December 31, 2017 and December 31, 2016, the Company had no other assets or liabilities that are subject to fair value methodology and estimation in accordance with U.S. GAAP.
Revenue Recognition
The Company follows Accounting Standards Update (“ASU”) 2009-13, Multiple-Deliverable Revenue Arrangements, and ASU 2010-17, Revenue Recognition-Milestone Method in connection with its accounting for collaboration arrangements. The Company’s revenues are generated primarily through arrangements which generally contain multiple elements, or deliverables, including licenses and R&D activities to be performed by the Company on behalf of the licensor or grantor. Payments to EyeGate under these arrangements typically include one or more of the following: (1) nonrefundable, upfront license fees, (2) funding of discovery research efforts on a full-time equivalent basis, (3) reimbursement of research, development and intellectual property costs, (4) milestone payments, and (5) royalties on future product sales.
When evaluating multiple element arrangements, Company management considers whether the deliverables under the arrangement represent separate units of accounting. This evaluation requires subjective determinations and requires Company management to make judgments about individual deliverables, including whether such deliverable is separable from the other aspects of the contractual relationship. In determining a unit of accounting, Company management evaluates certain criteria, including whether the deliverable has standalone value, based on the consideration of the relevant facts and circumstances for each arrangement. The consideration received is allocated among each separate unit of accounting using the relative selling price method, and the applicable revenue recognition criteria is applied to each separate unit.
The Company generally expects to recognize revenue attributable to a future license obtained on a straight-line basis over the Company’s contractual or estimated performance period, which is typically the term of the Company’s R&D obligation. If Company management cannot reasonably estimate when the Company’s performance obligation ends, then revenue is deferred until Company management can reasonably estimate when the performance obligation ends. The periods over which revenue should be recognized are subject to estimates by management and may change over the course of the R&D agreement. Such a change could have a material impact on the amount of revenue the Company records in future periods. At the inception of arrangements that include milestone payments, Company management evaluates whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone, (b) the consideration relates solely to past performance, and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement.
Company management evaluates factors such as the scientific, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required to achieve the respective milestone and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment. The Company has concluded that the clinical and development milestones pursuant to its R&D arrangements are substantive.
| F-14 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
2. Summary of Significant Accounting Policies - (continued)
The Company aggregates its milestones into four categories: (i) clinical and development milestones, (ii) the chemistry, manufacturing and controls (“CMC”) validation, (iii) regulatory milestones, and (iv) commercial milestones. Clinical and development milestones are typically achieved when a product candidate advances into a defined phase of clinical research or completes such phase or when a contractually specified clinical trial enrollment target is attained. CMC validation milestones are typically achieved when the validation paperwork is finalized. Regulatory milestones are typically achieved upon acceptance of the submission for marketing approval of a product candidate or upon approval to market the product candidate by the FDA or other global regulatory authorities. For example, a milestone payment may be due to the Company upon the FDA’s acceptance of an NDA. Commercial milestones are typically achieved when an approved pharmaceutical product reaches certain defined levels of net sales by the licensee, such as when a product first achieves global sales or annual sales of a specified amount.
Revenues from clinical and development, CMC and regulatory milestone payments (if the milestones are deemed substantive and the milestone payments are nonrefundable) are recognized upon successful accomplishment of the milestones. Revenue from commercial milestone payments are accounted for as royalties and are recorded as Revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
Payments or reimbursements resulting from the Company’s R&D activities are recognized as the services are performed and are presented on a gross basis so long as there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collection of the related receivable is reasonably assured. Amounts received prior to satisfying the above revenue recognition criteria are recorded as Deferred Revenue on the Consolidated Balance Sheet.
On July 9, 2015, the Company entered into an exclusive, worldwide licensing agreement with a subsidiary of Valeant Pharmaceuticals International, Inc. (“Valeant”), through which the Company granted to Valeant an exclusive, worldwide commercial and manufacturing right to the Company’s EGP-437 Product in the field of anterior uveitis, as well as a right of last negotiation to license its EGP-437 Product for indications other than anterior uveitis (the “Valeant Agreement”). There are four principal R&D milestones under the Valeant Agreement: (i) the Phase 3 Clinical Trial, (ii) the Endothelial Cell Count Safety Trial (a trial to determine that treatment has not adversely affected a patient’s corneal endothelial cell density), (iii) the CMC Validation, and (iv) the New Drug Application, or “NDA”, filing with the FDA (collectively, the “Four Milestones”, and each individually, a “Milestone”). Under the Valeant Agreement, Valeant paid to the Company an initial upfront payment, and the Company is eligible to receive certain other payments, upon and subject to the achievement of certain specified development and commercial progress of the EGP-437 Product for the treatment of anterior uveitis. The Company received the initial up-front payment in 2015, which it recorded as Deferred Revenue on its Consolidated Balance Sheet, and later in 2015 began receiving certain additional payments, based on R&D progress, to continue over several years. The Company receives payments both when it crosses certain thresholds on the way to each Milestone (each, a “Progress Payment”), as well as once it achieves each Milestone. The Company is entitled to retain all of these payments. The Company defers each Progress Payment, capitalizes each payment on its Consolidated Balance Sheet as Deferred Revenue, and recognizes these payments in the aggregate as Revenue once it achieves the Milestone to which the Progress Payment relates. The Company recognizes the initial upfront payment as Revenue ratably as it completes each of the Four Milestones, the amount recognized being the total upfront payment times the percentage represented by the proportionate share of fair value of each Milestone relative to the total fair value of all Milestones. Accordingly, the Deferred Revenue account on the Condensed Consolidated Balance Sheet is reduced as Revenue is recognized in the Consolidated Statement of Operations and Comprehensive Loss. Effective with the adoption of ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), the Company will begin recognizing Revenue with respect to the Valeant Agreement Progress Payments in the first quarter of 2018.
On February 21, 2017, the Company entered into another exclusive, worldwide licensing agreement with a subsidiary of Valeant (the “New Valeant Agreement”), through which the Company granted Valeant exclusive, worldwide commercial and manufacturing rights to its EGP-437 Product in the field of ocular iontophoretic treatment for post-operative ocular inflammation and pain in ocular surgery patients (the “New Field”). Under the New Valeant Agreement, Valeant paid the Company an initial upfront payment of $4.0 million, and the Company is eligible to receive milestone payments totaling up to approximately $99.0 million, upon and subject to the achievement of certain specified developmental and commercial progress of the EGP-437 Product for the New Field. The Company has received milestone payments totaling $3.234 million through December 31, 2017. In accordance with its revenue recognition policy, the initial upfront payment and milestone payments have been recorded as Deferred Revenue. Effective with the adoption of ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), the Company will begin recognizing Revenue with respect to the New Valeant Agreement Progress Payments in the first quarter of 2018. In addition, the Company is eligible under the New Valeant Agreement to receive royalties based on a specified percent of net sales of its EGP-437 Product for the New Field throughout the world, subject to adjustment in certain circumstances.
| F-15 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
2. Summary of Significant Accounting Policies - (continued)
The Company received government grant funds from two sources: the U.S. Department of Defense (“DoD”) and the National Science Foundation (“NSF”). The Company was paid by the DoD after it performed specified, agreed-upon research, and it recorded these grant funds as Revenue as it performed the research. The Company was generally paid by the NSF before it performed specified, agreed-upon research. The Company recorded these NSF funds on its Consolidated Balance Sheet as Deferred Revenue when invoiced, and recognized these amounts as Revenue ratably as the research was performed, typically over a six-month period.
The DoD and NSF each committed to grant funds to Jade for specified ocular therapeutic research activities (together, the “U.S. Government Grants”) to be conducted through 2017, which have been fully funded as of December 31, 2017. The Company recognizes grant funds as Revenue when it performs the activities specified by the terms of the grant and is entitled to the funds.
Recent Accounting Pronouncements
In November 2016, the FASB issued ASU No. 2016-18, Restricted Cash, which clarifies guidance and presentation related to restricted cash in the statement of cash flows, including stating that restricted cash should be included within cash and cash equivalents on the statement of cash flows. The standard is effective for fiscal years beginning after December 15, 2017, with early adoption permitted, and is to be applied retrospectively. The Company adopted this standard effective with these financial statements. Such adoption did not have a material effect on its financial statements and related disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases (“ASU 2016-02”), which is effective for fiscal years, and interim periods within those years, beginning after December 15, 2018, with early adoption permitted. Under ASU 2016-02, lessees will be required to recognize for all leases at the commencement date a lease liability, which is a lessee’s obligation to make lease payments arising from a lease measured on a discounted basis, and the right-to-use assets, which are asset that represents the lessee’s right to use or control the use of a specified asset for the lease term. The Company does not expect to early adopt this standard and currently has leases (see Note 11) that will be in place at the effective date. The Company is currently evaluating the effect that the new guidance will have on its financial statements and related disclosures.
In March 2016, the FASB issued an ASU No. 2016-09, Compensation- Stock Compensation (“ASU 2016-09”), which identifies areas for simplification involving several aspects of accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, an option to recognize gross stock compensation expense with actual forfeitures recognized as they occur, as well as certain classifications on the statement of cash flows. This update is effective for fiscal years beginning after December 15, 2016 including interim periods within that reporting period, with early adoption permitted. The Company has adopted the provisions of ASU 2016-09 in the first quarter of 2017 and the adoption of this guidance did not have a material impact on its consolidated financial statements. The guidance requires the recognition in the income statement of the income tax effects of vested or settled awards. Further, the guidance requires that the recognition of anticipated tax windfalls/shortfalls be excluded in the calculation of assumed proceeds when applying the treasury stock method. The guidance also allows for the employer to repurchase more of an employee’s shares for tax withholding purposes and not classify the award as a liability that requires valuation on a mark-to-market basis. In addition, the guidance allows for a policy election to account for forfeitures as they occur rather than on an estimated basis.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”), as subsequently amended, that outlines a single comprehensive model for entities to use in accounting for revenue arising from contracts with customers and supersedes most recent current revenue recognition guidance, including industry-specific guidance. The core principle of the revenue model is that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance also specifies the accounting for certain incremental costs of obtaining a contract, and costs to fulfill a contract with a customer. Entities have the option of applying either a full retrospective approach to all periods presented, or a modified approach that reflects differences prior to the date of adoption as an adjustment to equity. In April 2015, the FASB deferred the effective date of this guidance until January 1, 2018. The Company is not early adopting this standard. The Company’s sole revenue activities currently relate to the Valeant Agreements and its U.S. Government Grants.
The Company completed its implementation analysis, including identification of revenue streams and reviews of customer contracts under ASU 2014-09’s framework. The analysis included reviewing current accounting policies and practices to identify potential differences that would result from applying the requirements under this new standard. The Company reviewed its contracts with Valeant. ASU 2014-09 requires increased disclosure, which in turn requires certain new processes. The Company is opting to use the modified retrospective transition method, meaning the cumulative effect of applying the new guidance will be recognized at the date of initial application as an adjustment to the opening accumulated deficit balance, and thus on January 1, 2018, will record a reduction to its opening accumulated deficit balance of approximately $9.5 million. The Company will continue to recognize revenue over time in 2018 as performance obligations are met.
| F-16 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
3. Property and Equipment
Property and equipment at December 31, 2017 and 2016 consists of the following:
|
Estimated Useful Life (Years) |
2017 | 2016 | ||||||||||
| Laboratory Equipment | 3 | $ | 42,576 | $ | 42,576 | |||||||
| Office Furniture | 5 | 14,430 | - | |||||||||
| Leasehold Improvements | 2 | 22,569 | - | |||||||||
| 79,575 | 42,576 | |||||||||||
| Less Accumulated Depreciation | 23,824 | 4,536 | ||||||||||
| $ | 55,751 | $ | 38,040 | |||||||||
Depreciation expense was $19,288 and $5,185 for the years ended December 31, 2017 and 2016, respectively.
4. Accrued Expenses
Accrued expenses consist of the following:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Clinical Trials | $ | 807,322 | $ | 770,158 | ||||
| Payroll and Benefits | 788,551 | 668,802 | ||||||
| Professional Fees | 149,273 | 174,342 | ||||||
| Consulting | 57,487 | 44,983 | ||||||
| Short-Term Portion of Capital Lease Obligation | 11,214 | 12,645 | ||||||
| Total Accrued Expenses | $ | 1,813,847 | $ | 1,670,930 | ||||
5. Debt
The Company has no indebtedness other than trade and accounts payable and capital lease obligations in the ordinary course of business as of the years ended December 31, 2017 and 2016.
| F-17 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
6. Capital Stock
On May 24, 2016, the Company entered into an At The Market Offering Agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC (the “Sales Agent”), to create an at the market equity program under which the Company can from time to time offer and sell up to 1,319,289 shares of its Common Stock through the Sales Agent. Effective June 26, 2016, the Company halted indefinitely all future offers and sales of its Common Stock pursuant to the ATM Agreement. On June 30, 2016, the Company closed on the sale of its equity securities in connection with a registered direct offering, described below, and as a result, the Company was restricted from issuing any shares pursuant to the ATM Agreement for a period of 90 days following the close of the ATM Agreement. This restriction lapsed on September 28, 2016. On February 21, 2017, the Company authorized the Sales Agent to restart sales under the ATM Agreement for maximum aggregate gross proceeds of up to $3,285,798. During the first quarter of 2017, the Company sold 642,150 shares of Common Stock under this agreement for total net proceeds to the Company from this offering, after deducting the placement agent fees and offering expenses, of approximately $1.8 million. No shares of Common Stock were sold pursuant to the ATM Agreement during the second, third or fourth quarters of 2017. On June 14, 2017, the Company closed on the sale of its equity securities in connection with a public offering, described below, and as a result, the Company is restricted from issuing any shares pursuant to the ATM Agreement for a period of twenty-four months following the closing date of the offering. However, this restriction is suspended for any sale of shares of Common Stock under the ATM Agreement that is above $3.00 per share.
On June 27, 2016, in connection with the issuance of 2,776.5 shares of Series A Preferred Stock in the Company’s registered direct offering, the Company filed a Certificate of Designation of Preferences, Rights and Limitations of Series A Preferred Stock with the Delaware Secretary of State. Each share of Series A Preferred Stock has a stated value of $1,000 and is convertible into shares of the Company’s Common Stock at any time at the holder’s option at an initial conversion price of $2.25. The holder, however, would be prohibited from converting shares Preferred Stock into shares of Common Stock if, as a result of such conversion, the holder, together with its affiliates, would own more than 4.99% of the shares of the Company’s shares of Common Stock then issued and outstanding, which may be increased to 9.99% in certain circumstances. In the event of the Company’s liquidation, dissolution, or winding up, holders of Series A Preferred Stock would receive a payment equal to $0.01 per share of Series A Preferred Stock before any proceeds are distributed to the holders of shares of Common Stock. Shares of Series A Preferred Stock generally had no voting rights, except as required by law and except that the consent of holders of a majority of the outstanding Series A Preferred Stock will be required to amend any provision of the Company’s certificate of incorporation that would have a materially adverse effect on the rights of the holders of the Series A Preferred Stock. Shares of Series A Preferred Stock were not entitled to receive any dividends, unless and until specifically declared by the Company’s Board of Directors, and ranked:
| · | senior to all of the Company’s Common Stock to the extent of its liquidation preference of $0.01; |
| · | senior to any class or series of the Company’s capital stock hereafter created specifically ranking by its terms junior to the Series A Preferred Stock to the extent of its liquidation preference of $0.01; |
| · | senior to all of the Company’s outstanding warrants; and |
| · | on parity to any class or series of the Company’s capital stock hereafter created specifically ranking by its terms on parity with the Series A Preferred Stock. |
On June 30, 2016, the Company completed a registered direct offering of 441,000 shares of Common Stock and 2,776.5 shares of Series A Preferred stock (convertible into 1,234,000 shares of Common Stock), along with a concurrent private placement of warrants. Concurrently with the closing of the registered direct offering, the holder elected to convert 123.75 shares of Series A Preferred Stock into 55,000 shares of Common Stock. The total net proceeds to the Company from this offering, after deducting the placement agent fees and offering expenses, were approximately $3.4 million. Additionally, the investor received, for each share of Common Stock, or for each share of Common Stock issuable upon conversion of a share of Series A Preferred Stock purchased in the registered direct offering, warrants to purchase one-half of a share of Common Stock at an exercise price of $3.50 per share, aggregating warrants to purchase 837,500 shares of Common Stock. The warrants issued to the investor were initially exercisable six months following issuance, and terminate five years following the initial exercise date (December 30, 2016). In addition, the Company issued to the Sales Agent warrants to purchase 33,500 shares of Common Stock. The warrants and the shares of Common Stock underlying the warrants issued in this offering have not been registered under the Securities Act, or applicable state securities laws. During the year ended December 31, 2016, the holder of the Series A Preferred Stock converted all 2,776.5 shares of preferred stock into 1,234,000 shares of Common Stock.
| F-18 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
6. Capital Stock - (continued)
On June 14, 2017, the Company completed a public offering of 5,336,667 shares of Common Stock and 1,995 shares of Series B Preferred Stock (convertible into 1,330,000 shares of Common Stock), along with warrants to purchase 6,666,667 shares of Common Stock. Concurrently with the closing of the public offering, a holder elected to convert 675 shares of Series B Preferred Stock into 450,000 shares of Common Stock. Subsequently, on June 15, 2017, a holder converted 720 shares of Series B Preferred stock into 480,000 shares of Common Stock. The total net proceeds to the Company from the offering, after deducting the placement agent fees and offering expenses, were approximately $8.8 million. Additionally, the investors received, for each share of Common Stock, or for each share of Common Stock issuable upon conversion of a share of Series B Preferred Stock purchased in the public offering, warrants to purchase one share of Common Stock at an exercise price of $1.50 per share, which totaled warrants to purchase an aggregate of 6,666,667 shares of Common Stock. The warrants issued to investors became initially exercisable immediately upon issuance and terminate on June 14, 2022, five years following the date of issuance.
At each of December 31, 2017 and December 31, 2016, the Company had 100,000,000 authorized shares of Common Stock, $0.01 par value, of which 17,257,255 and 10,130,883 shares, respectively, were outstanding. At each of December 31, 2017 and December 31, 2016, the Company had 9,995,828 and 9,997,223 authorized shares of Preferred Stock, $0.01 par value, respectively, of which 3,750 shares were designated as Series A Preferred Stock and 0 shares are issued and outstanding, and 10,000 shares were designated as Series B Preferred Stock, and 600 and 0 shares, respectively, are issued and outstanding. The reduction in shares of authorized Preferred Stock is a result of 1,395 shares of Series B Preferred Stock, which were converted to Common Stock and retired during the year ended December 31, 2017. At each of December 31, 2017 and December 31, 2016, there were 0 shares of Common Stock underlying the outstanding shares of Series A Preferred Stock, and 400,000 and 0 shares of Common Stock underlying the outstanding shares of Series B Preferred Stock, respectively.
7. Warrants
At December 31, 2017 and 2016, the following warrants were outstanding:
|
Number of Awards |
Weighted Average Exercise Price |
Weighted Average Remaining Term in Years |
||||||||||
| Outstanding at December 31, 2015 | 1,983,673 | $ | 9.18 | 5.32 | ||||||||
| Issued | 871,000 | 1 | $ | 3.50 | 2 | 4.49 | ||||||
| Forfeited | (1,937 | ) | $ | 9.18 | 4.82 | |||||||
| Outstanding at December 31, 2016 | 2,852,736 | $ | 7.45 | 4.26 | ||||||||
| Issued | 6,666,667 | 3 | $ | 1.50 | 4 | 4.45 | ||||||
| Forfeited | (63,442 | ) | $ | 6.00 | 7.13 | |||||||
| Outstanding at December 31, 2017 | 9,455,961 | $ | 3.26 | 4.23 | ||||||||
| 1 | Consists of 1,742,000 warrants to purchase 837,500 shares of Common Stock issued to the investor, and 33,500 warrant shares issued to the Sales Agent, in connection with the Company’s registered direct offering on June 30, 2016. |
| 2 | Warrant exercise price for a full share of Common Stock. Each warrant issued is for the purchase of one-half of a share of Common Stock. |
| 3 | Consists of 6,666,667 warrants to purchase 6,666,667 shares of Common Stock issued in connection with the Company’s public offering on June 14, 2017. |
| 4 | Warrant exercise price for a full share of Common Stock. |
All of the warrant agreements provide for a cashless exercise in the event a registration statement covering the issuance of the shares of common stock underlying the warrants is not effective, whereby the number of warrants to be issued will be reduced by the number of shares which could be purchased from the proceeds of the exercise of the respective warrant. The outstanding warrants expire from 2020 through 2025.
| F-19 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
8. Stockholders’ Notes Receivable
In 2007, a Stockholder of the Company was issued various promissory notes totaling $58,824 for the sale of Common Stock. The notes were full recourse and collateralized by the shares of Common Stock sold. The amended notes bore compound interest at 0.93% effective October 1, 2012, and as of October 1, 2016 these notes had matured.
On September 5, 2017, these notes were forgiven by the Company in the amount of $91,054, which included accrued interest of $32,230. These amounts are recorded in General and Administrative on the Consolidated Statements of Operations and Comprehensive Loss.
9. Equity Incentive Plan
In 2005, the Company approved the 2005 Equity Incentive Plan (the “2005 Plan”). The 2005 Plan provides for the granting of options, restricted stock or other stock-based awards to employees, officers, directors, consultants and advisors. During 2010, the maximum number of shares of Common Stock that may be issued pursuant to the 2005 Plan was increased to 891,222 shares. The Board of Directors (the “Board”) is responsible for administration of the 2005 Plan. The Company’s Board determines the term of each option, the option exercise price, the number of shares for which each option is granted and the rate at which each option is exercisable. Incentive stock options may be granted to any officer or employee at an exercise price per share of not less than the fair value per common share on the date of the grant (not less than 110% of fair value in the case of holders of more than 10% of the Company’s voting stock) and with a term not to exceed ten years from the date of the grant (five years for incentive stock options granted to holders of more than 10% of the Company’s voting stock). Nonqualified stock options may be granted to any officer, employee, consultant or director at an exercise price per share of not less than the par value per share. Following adoption of the 2014 Equity Incentive Plan (the “2014 Plan”), no further grants were made under the 2005 Plan.
The Company’s Board adopted the 2014 Plan and the Employee Stock Purchase Plan (the “ESPP”), and the Company’s Stockholders approved the 2014 Plan and the ESPP Plan in February 2015. On December 27, 2017, the Company issued 53,477 shares to employees pursuant to the ESPP. As of December 31, 2017, the maximum number of shares of Common Stock that may be issued pursuant to the 2014 Plan and the ESPP is 1,690,123 and 117,090 shares, respectively.
In January 2017, the number of shares of common stock issuable under the 2014 Plan automatically increased by 405,235 shares pursuant to the terms of the 2014 Plan. Additionally, in June 2017, the number of shares of common stock issuable under the 2014 Plan was increased by 250,000 shares and issuable under the ESPP was increased by 100,000 shares, as approved by the Company’s Stockholders. These additional shares are included in the total of 1,690,123 shares issuable under the 2014 Plan and 117,090 shares issuable under the ESPP.
The following is a summary of stock option activity for the twelve months ended December 31, 2017 and 2016:
| Number of Options |
Weighted- Exercise Price |
Weighted-Average Contractual Life |
||||||||||
| Outstanding at December 31, 2015 | 1,277,367 | $ | 2.75 | 4.94 | ||||||||
| Granted | 377,771 | 2.74 | 9.79 | |||||||||
| Exercised | (86,765 | ) | 0.65 | |||||||||
| Forfeited | (58,662 | ) | 3.31 | |||||||||
| Outstanding at December 31, 2016 | 1,509,711 | $ | 2.85 | 5.04 | ||||||||
| Granted | 568,450 | 1.39 | 9.41 | |||||||||
| Exercised | (61,078 | ) | 0.67 | |||||||||
| Expired | (92,630 | ) | 2.10 | |||||||||
| Forfeited | (31,450 | ) | 2.99 | |||||||||
| Outstanding at December 31, 2017 | 1,893,003 | $ | 2.49 | 5.40 | ||||||||
| Exercisable at December 31, 2017 | 1,208,450 | $ | 2.73 | 4.87 | ||||||||
| Vested and Expected to Vest at December 31, 2017 | 1,208,450 | $ | 2.73 | 4.87 | ||||||||
| F-20 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
9. Equity Incentive Plan - (continued)
The Company originally estimated the volatility of its Common Stock based on the average of published volatilities contained in the most recent audited financial statements of other SEC reporting companies in industries similar to that of the Company. Effective July 1, 2016, the Company determined that the prior methodology for measuring the volatility of its Common Stock was no longer the best estimate of volatility and the Company will measure volatility using its Common Stock volatility. The Company believes that the public market for its Common Stock is the best measure to use as an input in the option pricing model. All future grants of stock options will use the Company’s historic Common Stock volatility.
During the years ended December 31, 2017 and December 31, 2016, the Board approved the grant of options to purchase 568,450 and 377,771 shares of its Common Stock, respectively. All option grants were pursuant to the 2014 Plan. In general, options granted under the 2014 Plan vest 33.33% on the one-year anniversary of the grant date, and the remainder ratably over the 24-month period following the one-year anniversary.
On February 6, 2017, the Board approved the grant of 104,000 shares of restricted stock to eight employees. These vest 33.33% on the one-year anniversary of the grant date, and the remainder ratably over the 24-month period following the one-year anniversary. As of December 31, 2017, none of these shares were vested and 1,000 shares were cancelled due to an employee termination.
For the twelve months ended December 31, 2017 and 2016, the fair value of each option grant has been estimated on the date of grant using the Black-Scholes Option Pricing Model with the following weighted-average assumptions:
| 2017 | 2016 | |||||||
| Risk-Free Interest Rate | 1.82 | % | 1.82 | % | ||||
| Expected Life | 7.25 years | 7.00 years | ||||||
| Expected Volatility | 170 | % | 99 | % | ||||
| Expected Dividend Yield | 0 | % | 0 | % | ||||
The total stock-based compensation expense for employees and non-employees is included in the accompanying Consolidated Statements of Operations and as follows:
| Year Ended December 31 | ||||||||
| 2017 | 2016 | |||||||
| Research and Development | $ | 193,688 | $ | 46,288 | ||||
| General and Administrative | 676,620 | 464,707 | ||||||
| $ | 870,308 | $ | 510,995 | |||||
The fair value of options granted for the twelve months ended December 31, 2017 and December 31, 2016 was approximately $640,000 and $758,000, respectively. The fair value of restricted stock granted for the twelve months ended December 31, 2017 and December 31, 2016 was approximately $158,000 and $0, respectively. As of December 31, 2017 and December 31, 2016, there was approximately $975,000 and $984,000 of total unrecognized compensation expense related to unvested stock-based compensation arrangements granted, which cost is expected to be recognized over a weighted average period of 2.03 and 2.43 years, respectively. The aggregate intrinsic value of stock options outstanding and exercisable at December 31, 2017 and December 31, 2016 was approximately $191,000 and $544,000, respectively. The intrinsic value of stock options exercised during 2017 and 2016 was approximately $78,000 and $207,000, respectively.
At December 31, 2017, there were options to purchase 116,411 shares of Common Stock available for grant under the 2014 Plan. During the year ended December 31, 2017, 53,477 shares were issued pursuant to the Company’s ESPP at a discounted price of $1.01.
| F-21 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
10. Income Taxes
The components of loss before income taxes are as follows:
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Domestic | $ | (15,102,521 | ) | $ | (13,831,191 | ) | ||
| Foreign | 542,631 | 487,754 | ||||||
| Total | $ | (14,559,890 | ) | $ | (13,343,437 | ) | ||
The components of income tax benefit are as follows:
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Deferred Taxes: | ||||||||
| Federal | $ | (1,160,312 | ) | $ | - | |||
| State | (181,661 | ) | - | |||||
| Total Deferred Taxes | $ | (1,341,973 | ) | $ | - | |||
| Income Tax Benefit | $ | (1,341,973 | ) | $ | - | |||
The difference between the effective rate reflected in the provision for income taxes on loss before taxes and the amounts determined by applying the applicable statutory U.S. tax rate are analyzed below:
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| United States Federal Income Tax Rate | 34.00 | % | 34.00 | % | ||||
| State Taxes, Net of Federal Benefit | 4.68 | % | 1.84 | % | ||||
| Permanent Differences | (2.67 | )% | (2.47 | )% | ||||
| Change in Valuation Allowance | 46.88 | % | (35.02 | )% | ||||
| Research and Development Credits | 1.97 | % | 2.95 | % | ||||
| Tax Rate Differential | 0.01 | % | 0.13 | % | ||||
| Federal Rate Change | (71.74 | )% | 0.00 | % | ||||
| Other | (3.91 | )% | (1.43 | )% | ||||
| Effective Tax Rate | 9.22 | % | 0.00 | % | ||||
The Company’s deferred tax assets and liabilities consist of the following:
| 2017 | 2016 | |||||||
| Net Deferred Tax Liability: | ||||||||
| Net Operating Loss Carryforwards | $ | 12,624,253 | $ | 18,146,381 | ||||
| Research and Development Credit Carryforwards | 2,002,640 | 1,640,669 | ||||||
| Capitalized Research and Development | 5,172,541 | 6,398,050 | ||||||
| Nonqualified Stock Option | 552,096 | 323,832 | ||||||
| Depreciation and Amortization | (82 | ) | 3,478 | |||||
| Cash Versus Accrual Adjustments | 4,459,785 | 4,387,806 | ||||||
| Total Deferred Tax Assets | 24,811,233 | 30,900,216 | ||||||
| Valuation Allowance | (24,075,539 | ) | (30,900,216 | ) | ||||
| Net Deferred Tax Asset | 735,694 | - | ||||||
| In-Process Research and Development | (919,617 | ) | (1,525,896 | ) | ||||
| Net Deferred Tax Liability | $ | (183,923 | ) | $ | (1,525,896 | ) | ||
| F-22 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
10. Income Taxes - (continued)
On December 22, 2017, the TCJA was enacted and significantly revised the U.S. income tax law. The TCJA includes changes, which reduce the corporate income tax rate from 34% to 21% for years beginning after December 31, 2017 and transitions the U.S. towards a territorial tax system where U.S. shareholders of certain foreign subsidiaries would be subject to a one-time transition tax on unrepatriated accumulated foreign earnings.
U.S. GAAP requires re-measurement of U.S. deferred tax assets and liabilities to reflect the impact of tax rate reduction in the period that includes enactment date. As a result, the Company had revalued its deferred taxes assets and liabilities to 21% in the December 31, 2017 financial statements and the impact was offset by the Company’s valuation allowance. The Company has reflected the associated impact in the reconciliation of the Company’s effective tax rate above.
On December 22, 2017, Staff Accounting Bulletin No. 118 (“SAB 118”) was issued and allows a company to recognize provisional amounts when it does not have the necessary information available, prepared or analyzed, including computations, in reasonable detail to complete its accounting for the change in tax law. SAB 118 provides for a measurement of up to one year from the date of enactment. The Company has not yet fully completed its analysis of the TCJA, however based on the total unrepatriated accumulated foreign earnings, the Company does not believe any additional income taxes are due as any income, if determined, will be fully offset by the Company’s net operating losses.
As of December 31, 2017, the Company has federal, and state net operating loss carryforwards of approximately $45,494,000 and $33,258,000, respectively, to offset future federal and state taxable income, which expire at various times through 2037. The Company has foreign net operating loss carryforwards of $3,201,000 as of December 31, 2017, which can be carried forward indefinitely. As of December 31, 2017, the Company also has federal, state and foreign research and development tax credit carryforwards of approximately $1,601,000, $478,000, and $25,000, respectively, to offset future income taxes, which expire at various times through 2037. The federal and state net operating loss and research tax credit carryforwards may be subject to the limitations provided in the Internal Revenue Code (“IRC”) Sections 382 and 383. A portion of the federal net operating loss attributable to Jade is subject to a Section 382 limitation. Jade’s carryover of its research and development credits will be subject to the Section 383 limitation.
The Company files United States federal income tax returns and income tax returns in the Commonwealth of Massachusetts, Utah, and New Jersey, as well as foreign tax returns for its subsidiary in France. The Company is not under examination by any jurisdiction for any tax year.
The Company has recorded a valuation allowance against its United States deferred tax assets in each of the years ended December 31, 2017, and 2016 because the Company’s management believes that it is more likely than not that these assets will not be realized. The valuation allowance decreased by approximately $6,800,000 and increased by approximately $4,810,000 during the years ended December 31, 2017 and 2016, respectively, primarily as a result of adjustments for accrual to cash basis items and capitalized research and development expenses. Additionally, during the year ended December 31, 2017, deferred tax assets and liabilities were reduced to reflect the new federal tax rate under the TCJA and the Company partially released its valuation allowance against its previously recorded deferred tax assets where future reversals of deductible temporary differences, such as those from the Company’s indefinite-lived in-process research and development, can offset taxable temporary differences from future net operating loss carryforwards due to their indefinite carryforward period under new tax law.
As of December 31, 2017 and 2016, the Company had no unrecognized tax benefits or related interest and penalties accrued. The Company will recognize interest and penalties related to tax positions in income tax expense. The Company has not, as yet, conducted a study of R&D credit carryforwards. This study may result in an adjustment to the Company’s R&D credit carryforwards, however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position.
The net operating loss and tax credit carryforwards are subject to review by the Internal Revenue Service in accordance with the provisions of Section 382 of the Internal Revenue Code. Under this Internal Revenue Code section, substantial changes in the Company’s ownership may limit the amount of net operating loss carryforwards that could be utilized annually in the future to offset the Company’s taxable income. Specifically, this limitation may arise in the event of a cumulative change in ownership of the Company of more than 50% within a three-year period. Any such annual limitation may significantly reduce the utilization of the Company’s net operating loss carryforwards before they expire. The closing of the Company’s initial public offering, alone or together with transactions that have occurred or that may occur in the future, may trigger an ownership change pursuant to Section 382, which could limit the amount of research and development tax credit and net operating loss carryforwards that could be utilized annually in the future to offset the Company’s taxable income, if any. Any such limitation as the result of the Company’s additional sales of common stock by the Company could have a material adverse effect on the Company’s results of operations in future years.
| F-23 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
11. Commitments and Contingencies
Leases
The Company is a party to a real property operating lease for the rental of office space in Waltham, Massachusetts of up to 4,516 square feet, that is used for its corporate headquarters. This lease terminates in December 2019. On July 6, 2016, the Company entered into a real property operating lease for office and laboratory space of approximately 2,300 square feet in Salt Lake City, Utah. This lease terminates in June 2019. Estimated minimum lease payments for the years ended December 31, 2018 and 2019 are $170,000 and $144,000, respectively.
The Company is a party to two nominal equipment capital lease agreements, one for a three-year term and one for a two-year term, for the use of scientific instruments in its Salt Lake City laboratory.
License Agreements
The Company is a party to six license agreements as described below. Four of the six license agreements require the Company to pay royalties or fees to the licensor based on Revenue related to the licensed technology, and the agreements with Valeant require Valeant to pay royalties to the Company based on revenue related to the licensed technology.
On February 15, 1999, the Company entered in to an exclusive worldwide license agreement with the University of Miami School of Medicine to license technology relating to the Company’s EyeGate® II Delivery System. This agreement, which was amended in December 2005, requires the Company to pay to the University of Miami an annual license fee of $12,500. This license also requires payments to the University of Miami upon the Company’s achievement of certain milestones. Unless terminated pursuant to the license agreement, this license will expire 12 years after the date of the first commercial sale of a product containing the licensed technology.
On July 23, 1999, the Company entered into a perpetual Transaction Protocol agreement with Francine Behar-Cohen to acknowledge the Company’s right to use certain patents that Ms. Behar-Cohen had certain ownership rights with respect to and which are used in the Company’s EGP-437 Combination Product. The agreement also provides for the Company to pay Ms. Behar-Cohen a fee based on a percentage of the pre-tax turnover generated from sales of the Company’s EGP-437 Combination Product relating to its inclusion of the EyeGate® II Delivery System. The fees due under the agreement are required to be paid until January 2018.
On September 12, 2013, Jade entered into an agreement with BioTime, Inc. granting to it the exclusive worldwide right to commercialize cross-linked thiolated carboxymethyl hyaluronic acid (“CMHA-S”) for ophthalmic treatments in humans. The agreement calls for a license issue fee paid to BioTime of $50,000, and requires the Company (through its Jade subsidiary) to pay an annual fee of $30,000 and royalties to BioTime based on revenue relating to any product incorporating the CMHA-S technology. The agreement expires when patent protection for the CMHA-S technology lapses.
On July 9, 2015, the Company entered into an exclusive worldwide licensing agreement with a subsidiary of Valeant through which EyeGate has granted Valeant exclusive, worldwide commercial and manufacturing rights to its EGP-437 Product in the field of anterior uveitis, as well as a right of last negotiation to license the EGP-437 Product for other indications. Under the agreement, Valeant paid the Company an upfront payment of $1.0 million. The Company is eligible to receive milestone payments totaling up to $32.5 million, upon and subject to the achievement of certain specified developmental and commercial milestones. In addition, the Company is eligible to receive royalties based on a specified percent of net sales of the Product throughout the world, subject to adjustment in certain circumstances.
On June 17, 2016, the Company entered into an exclusive worldwide license agreement with the University of Utah Research Foundation to further the commercial development of the NASH technology, together with alkylated HA. The agreement calls for payments due to the University of Utah, consisting of a license grant fee of $15,000 due within 30 days of signing, and an annual licensing fee, initially $5,000, and escalating ratably up to $20,000 in 2021.
| F-24 |
EYEGATE PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2017
11. Commitments and Contingencies - (continued)
On February 21, 2017, the Company entered into an exclusive, worldwide licensing agreement with a subsidiary of Valeant (the “New Valeant Agreement”), through which the Company granted Valeant exclusive, worldwide commercial and manufacturing rights to its EGP-437 Product in the field of ocular iontophoretic treatment for post-operative ocular inflammation and pain in ocular surgery patients (the “New Field”). Under the New Valeant Agreement, Valeant paid the Company an initial upfront payment of $4.0 million, and the Company is eligible to receive milestone payments totaling up to approximately $99.0 million, upon and subject to the achievement of certain specified developmental and commercial progress of the EGP-437 Product for the New Field. In addition, the Company is eligible under the New Valeant Agreement to receive royalties based on a specified percent of net sales of its EGP-437 Product for the New Field throughout the world, subject to adjustment in certain circumstances.
12. Employee Benefit Plans
The Company has an employee benefit plan for its United States-based employees under Section 401(k) of the Internal Revenue Code. The Plan allows all eligible employees to make contributions up to a specified percentage of their compensation. Under the Plan, the Company may, but is not obligated to, match a portion of the employee contribution up to a defined maximum. The Company made no matching contribution for the years ended December 31, 2017 and 2016.
| F-25 |
Pursuant to the requirements of Section 13 and 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: March 2, 2018 | By: | /s/ Stephen From |
| Chief Executive Officer and President |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| Signature | Title | Date | ||
| /s/ Stephen From | President, Chief Executive Officer and Director | March 2, 2018 | ||
| Stephen From |
(principal executive officer) |
|||
| /s/ Sarah Romano | Chief Financial Officer | March 2, 2018 | ||
| Sarah Romano | (principal financial and accounting officer) | |||
| /s/ Paul Chaney | Chairman | March 2, 2018 | ||
| Paul Chaney | ||||
| /s/ Morton Goldberg | Director | March 2, 2018 | ||
| Morton Goldberg | ||||
| /s/ Praveen Tyle | Director | March 2, 2018 | ||
| Praveen Tyle | ||||
| /s/ Thomas E. Hancock | Director | March 2, 2018 | ||
| Thomas E. Hancock | ||||
| /s/ Bernard Malfroy-Camine | Director | March 2, 2018 | ||
| Bernard Malfroy-Camine |
EXHIBIT INDEX
The following exhibits are filed as part of this Annual Report on Form 10-K. Where such filing is made by incorporation by reference to a previously filed document, such document is identified.
| 101.INS* | XBRL Instance Document. | |
| 101.SCH* | XBRL Taxonomy Extension Schema Document. | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB* | XBRL Taxonomy Extension Labels Linkbase Document. | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document. |
| 1 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed March 7, 2015) and incorporated by reference thereto. |
| 2 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed February 20, 2015) and incorporated by reference thereto. |
| 3 | Previously filed as an exhibit to Amendment No. 2 to the Company’s Registration Statement on Form S-1 (filed August 29, 2014) and incorporated by reference thereto. |
| 4 | Previously filed as an exhibit to the Company’s Registration Statement on Form S-1 (filed July 30, 2014) and incorporated by reference thereto. |
| 5 | Previously filed as an exhibit to Amendment No. 7 to the Company’s Registration Statement on Form S-1 (filed December 24, 2014) and incorporated by reference thereto. |
| 6 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed July 10, 2015) and incorporated by reference thereto. |
| 7 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed August 5, 2015) and incorporated by reference thereto. |
| 8 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed June 27, 2016) and incorporated by reference thereto. |
| 9 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed May 25, 2016) and incorporated by reference thereto. |
| 10 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed April 29, 2016) and incorporated by reference thereto. |
| 11 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed December 12, 2016) and incorporated by reference thereto. |
| 12 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed February 6, 2017) and incorporated by reference thereto. |
| 13 | Previously filed as an exhibit to the Company’s Annual Report on Form 10-K (filed March 30, 2016) and incorporated by reference thereto. |
| 14 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed June 14, 2017) and incorporated by reference thereto. |
| 15 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed June 9, 2017) and incorporated by reference thereto. |
| 16 | Previously filed as an exhibit to Amendment No. 2 to the Company’s Registration Statement on Form S-1 (filed June 5, 2017) and incorporated by reference thereto. |
| 17 | Previously filed as an exhibit to the Company’s Annual Report on Form 10-K (filed February 23, 2017) and incorporated by reference thereto. |
| 18 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed November 29, 2017) and incorporated by reference thereto. |
| 19 | Previously filed as an exhibit to the Company’s Current Report on Form 8-K (filed January 4, 2018) and incorporated by reference thereto. |
| * | Filed herewith. |
| ** | This certification shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, or otherwise subject to the liability of that section, nor shall it be deemed to be incorporated by reference into any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934. |
| † | Confidential treatment requested as to portions of the exhibit. Confidential materials omitted and filed separately with the Securities and Exchange Commission. |
| # | Management contract or compensatory plan or arrangement. |