EXHIBIT 99.1
Published on November 9, 2017
Exhibit 99.1

Two Versatile Platforms Moving Towards Commercialization

Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncerta inties, including statements relating to the prospects for the Company’s EGP - 437 and OBG product candidates, for the timing and outcome of the Company’s clin ical trials, the potential approval to market the Company’s product candidates, and the Company’s capital needs. Actual events could differ materially fro m those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connectio n with, the presentation. Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful. The Company may consume mor e cash than it currently anticipates and faster than projected. Competitive products may reduce or eliminate the commercial opportunities of the Comp any ’s product candidates. If the U.S. Food and Drug Administration or foreign regulatory agencies determine that the Company’s product candidates do not meet saf ety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. Operating ex pen se and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the ti ming and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collabo rat ion opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay , s cale back or discontinue operations. Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Co mpany’s Annual Report on Form 10 - K filed with the SEC on February 23, 2017 or described in the Company’s other filings. The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s e xpe ctations, except as required by applicable law. 2 Forward Looking Statements
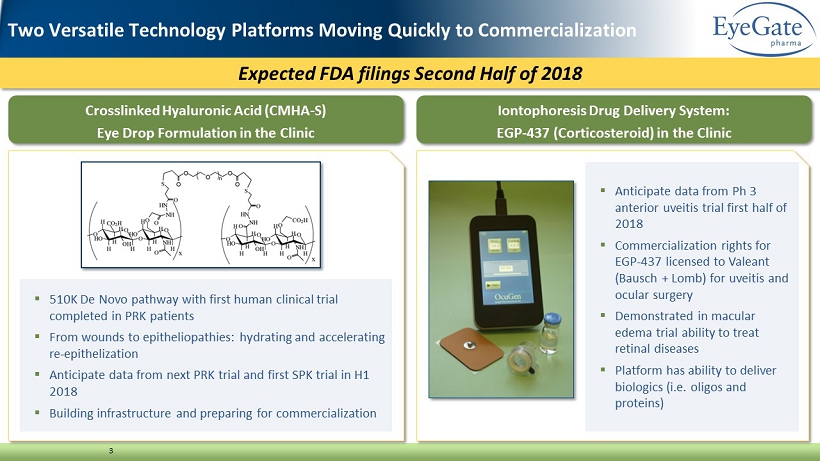
Expected FDA filings Second Half of 2018 3 Crosslinked Hyaluronic Acid (CMHA - S) Eye Drop Formulation in the Clinic Iontophoresis Drug Delivery System: EGP - 437 (Corticosteroid) in the Clinic Two Versatile Technology Platforms Moving Quickly to Commercialization ▪ 510K De Novo pathway with first human clinical trial completed in PRK patients ▪ From wounds to epitheliopathies: hydrating and accelerating re - epithelization ▪ Anticipate data from next PRK trial and first SPK trial in H1 2018 ▪ Building infrastructure and preparing for commercialization ▪ Anticipate data from Ph 3 anterior uveitis trial first half of 2018 ▪ Commercialization rights for EGP - 437 licensed to Valeant (Bausch + Lomb) for uveitis and ocular surgery ▪ Demonstrated in macular edema trial ability to treat retinal diseases ▪ Platform has ability to deliver biologics (i.e. oligos and proteins)
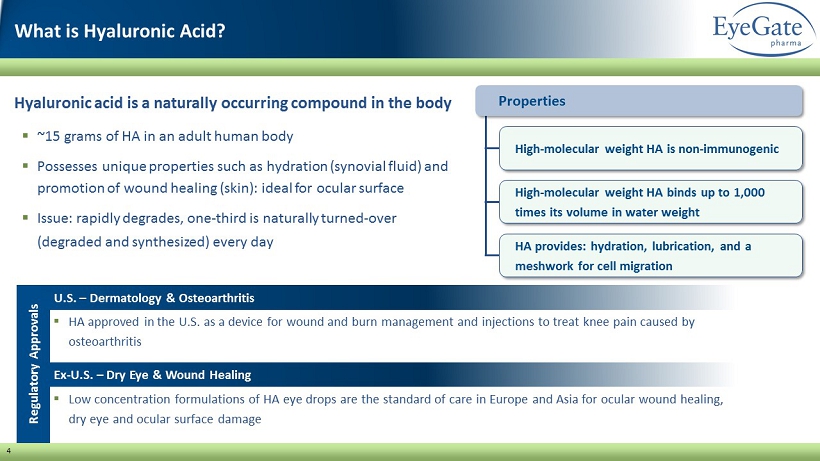
4 Hyaluronic acid is a naturally occurring compound in the body ▪ ~15 grams of HA in an adult human body ▪ Possesses unique properties such as hydration (synovial fluid) and promotion of wound healing (skin): ideal for ocular surface ▪ Issue: rapidly degrades, one - third is naturally turned - over (degraded and synthesized) every day ▪ HA approved in the U.S. as a device for wound and burn management and injections to treat knee pain caused by osteoarthritis Properties High - molecular weight HA is non - immunogenic High - molecular weight HA binds up to 1,000 times its volume in water weight HA provides: hydration, lubrication, and a meshwork for cell migration Regulatory Approvals ▪ Low concentration formulations of HA eye drops are the standard of care in Europe and Asia for ocular wound healing, dry eye and ocular surface damage U.S. – Dermatology & Osteoarthritis Ex - U.S. – Dry Eye & Wound Healing What is Hyaluronic Acid?
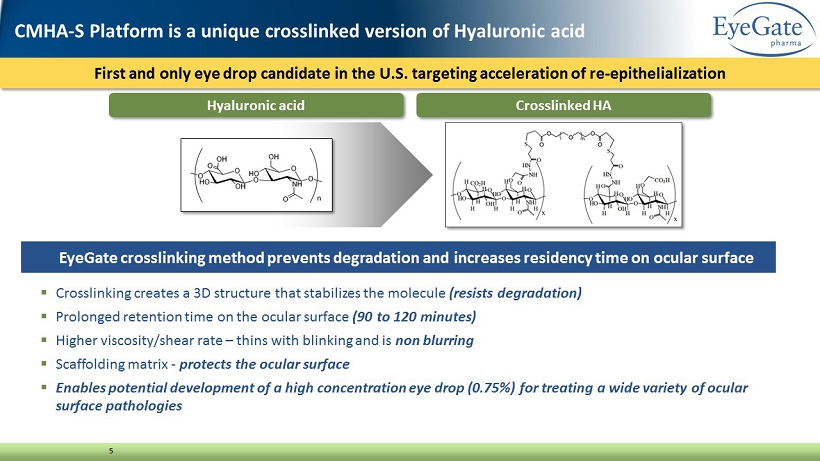
First and only eye drop candidate in the U.S. targeting acceleration of re - epithelialization 5 ▪ Crosslinking creates a 3D structure that stabilizes the molecule (resists degradation) ▪ Prolonged retention time on the ocular surface (90 to 120 minutes) ▪ Higher viscosity/shear rate – thins with blinking and is non blurring ▪ Scaffolding matrix - protects the ocular surface ▪ Enables potential development of a high concentration eye drop (0.75%) for treating a wide variety of ocular surface pathologies EyeGate crosslinking method prevents degradation and increases residency time on ocular surface Crosslinked HA Hyaluronic acid CMHA - S Platform is a unique crosslinked version of Hyaluronic acid
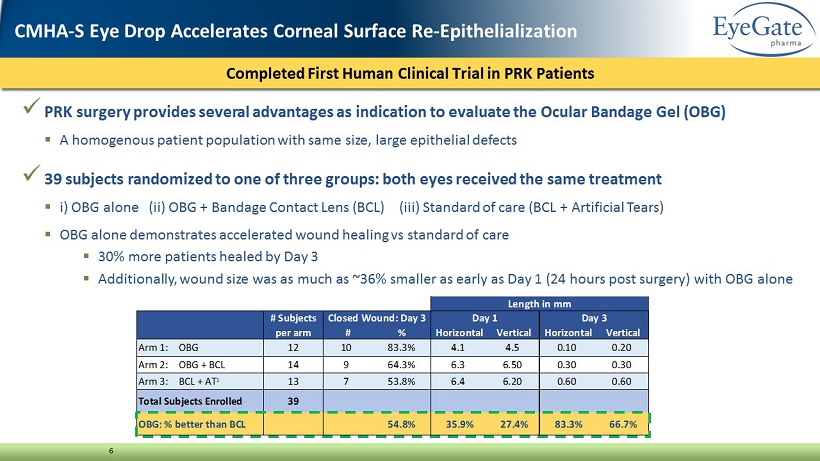
x PRK surgery provides several advantages as indication to evaluate the Ocular Bandage Gel (OBG) ▪ A homogenous patient population with same size, large epithelial defects x 39 subjects randomized to one of three groups: both eyes received the same treatment ▪ i ) OBG alone (ii) OBG + Bandage Contact Lens (BCL) (iii) Standard of care (BCL + Artificial Tears) ▪ OBG alone demonstrates accelerated wound healing vs standard of care ▪ 30% more patients healed by Day 3 ▪ Additionally, wound size was as much as ~36% smaller as early as Day 1 (24 hours post surgery) with OBG alone 6 First Human Clinical Trial Completed in PRK Patients targeting acceleration of re - epithelialization Completed First Human Clinical Trial in PRK Patients CMHA - S Eye Drop Accelerates Corneal Surface Re - Epithelialization # Subjects per arm # % Horizontal Vertical Horizontal Vertical Arm 1: OBG 12 10 83.3% 4.1 4.5 0.10 0.20 Arm 2: OBG + BCL 14 9 64.3% 6.3 6.50 0.30 0.30 Arm 3: BCL + AT 1 13 7 53.8% 6.4 6.20 0.60 0.60 Total Subjects Enrolled 39 OBG: % better than BCL 54.8% 35.9% 27.4% 83.3% 66.7% Length in mm Closed Wound: Day 3 Day 1 Day 3
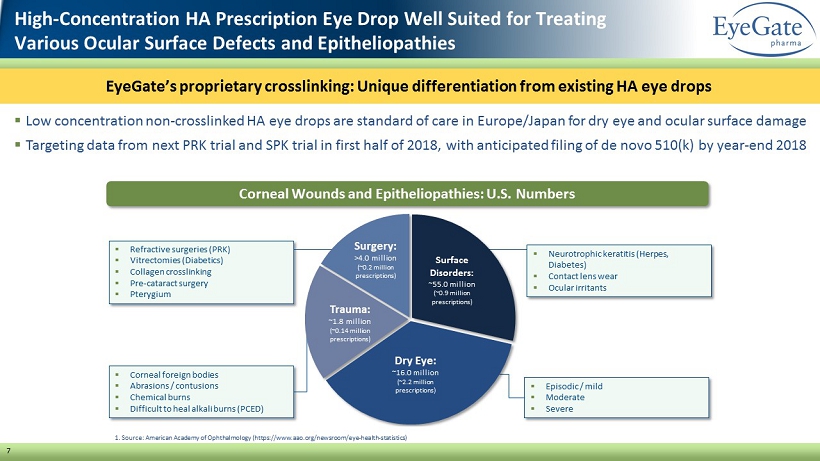
▪ Corneal foreign bodies ▪ Abrasions / contusions ▪ Chemical burns ▪ Difficult to heal alkali burns (PCED) 7 ▪ Low concentration non - crosslinked HA eye drops are standard of care in Europe/Japan for dry eye and ocular surface damage ▪ Targeting data from next PRK trial and SPK trial in first half of 2018, with anticipated filing of de novo 510(k) by year - end 20 18 ▪ Refractive surgeries (PRK) ▪ Vitrectomies (Diabetics) ▪ Collagen crosslinking ▪ Pre - cataract surgery ▪ Pterygium ▪ Neurotrophic keratitis (Herpes, Diabetes) ▪ Contact lens wear ▪ Ocular irritants ▪ Episodic / mild ▪ Moderate ▪ Severe Corneal Wounds and Epitheliopathies: U.S. Numbers Surface Disorders: ~55.0 million (~0.9 million prescriptions) Dry Eye: ~16.0 million (~2.2 million prescriptions) Trauma: ~1.8 million (~0.14 million prescriptions) Surgery : >4.0 million (~0.2 million prescriptions) 1. Source: American Academy of Ophthalmology (https://www.aao.org/newsroom/eye - health - statistics) High - Concentration HA Prescription Eye Drop Well Suited for Treating Various Ocular Surface Defects and Epitheliopathies EyeGate’s proprietary crosslinking: Unique differentiation from existing HA eye drops
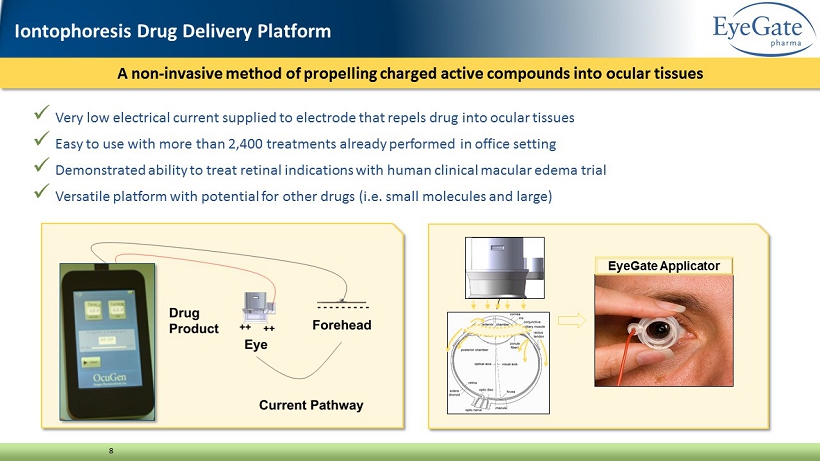
x Very low electrical current supplied to electrode that repels drug into ocular tissues x Easy to use with more than 2,400 treatments already performed in office setting x Demonstrated ability to treat retinal indications with human clinical macular edema trial x Versatile platform with potential for other drugs (i.e. small molecules and large) EyeGate Applicator A non - invasive method of propelling charged active compounds into ocular tissues 8 Iontophoresis Drug Delivery Platform
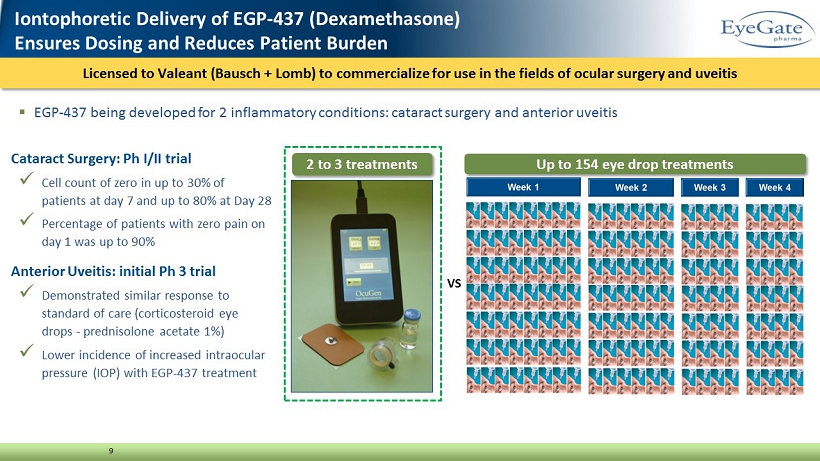
Licensed to Valeant (Bausch + Lomb) to commercialize for use in the fields of ocular surgery and uveitis 9 2 to 3 treatments Up to 154 eye drop treatments VS Iontophoretic Delivery of EGP - 437 (Dexamethasone) Ensures Dosing and Reduces Patient Burden ▪ EGP - 437 being developed for 2 inflammatory conditions: cataract surgery and anterior uveitis Cataract Surgery: Ph I/II trial x Cell count of zero in up to 30% of patients at day 7 and up to 80% at Day 28 x Percentage of patients with zero pain on day 1 was up to 90% Anterior Uveitis: initial Ph 3 trial x Demonstrated similar response to standard of care (corticosteroid eye drops - prednisolone acetate 1%) x Lower incidence of increased intraocular pressure (IOP) with EGP - 437 treatment
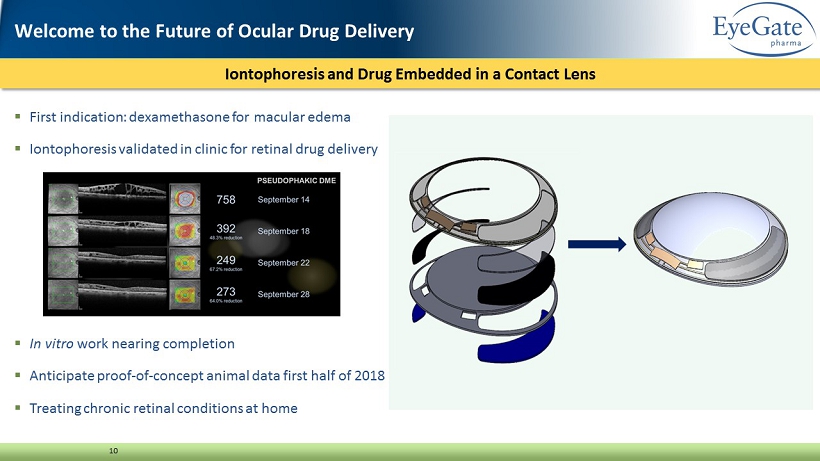
Iontophoresis and Drug Embedded in a Contact Lens 10 ▪ First indication: dexamethasone for macular edema ▪ Iontophoresis validated in clinic for retinal drug delivery ▪ In vitro work nearing completion ▪ Anticipate proof - of - concept animal data first half of 2018 ▪ Treating chronic retinal conditions at home Welcome to the Future of Ocular Drug Delivery
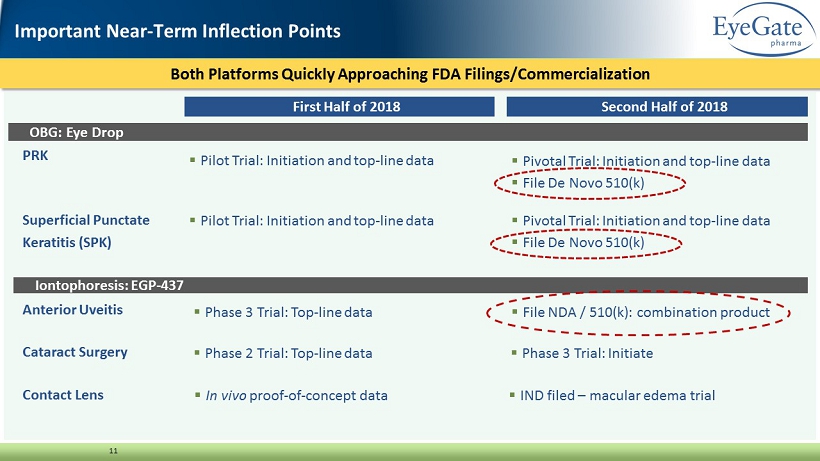
Both Platforms Quickly Approaching FDA Filings/Commercialization 11 PRK Superficial Punctate Keratitis (SPK) Important Near - Term Inflection Points First Half of 2018 Second Half of 2018 OBG: Eye Drop Iontophoresis: EGP - 437 ▪ Pilot Trial: Initiation and top - line data Anterior Uveitis Cataract Surgery Contact Lens ▪ Pilot Trial: Initiation and top - line data ▪ Pivotal Trial: Initiation and top - line data ▪ File De Novo 510(k) ▪ Pivotal Trial: Initiation and top - line data ▪ File De Novo 510(k) ▪ Phase 3 Trial: Top - line data ▪ File NDA / 510(k): combination product ▪ Phase 2 Trial: Top - line data ▪ Phase 3 Trial: Initiate ▪ In vivo proof - of - concept data ▪ IND filed – macular edema trial

12 Two Versatile Platforms Approaching Commercialization CMHA - S Platform ▪ Ocular Bandage Gel (Eye Drop): ▪ Anticipate De Novo 510(k) filing second half of 2018 for PRK and SPK ▪ Preparing for commercialization launch second half of 2019 ▪ Versatile platform with opportunities for other indications and products Iontophoresis Platform ▪ EGP - 437 nearing critical milestones ▪ Top - line data for 2 nd anterior uveitis Phase 3 trial expected first half of 2018 ▪ Top - line data for Phase 2 cataract surgery trial expected first half of 2018 ▪ NDA filing for anterior uveitis second half of 2018 ▪ Both indications licensed to Valeant (Bausch + Lomb) for commercialization ▪ Versatile platform with opportunities for other drugs and an at - home version of platform