EXHIBIT 99.1
Published on March 10, 2017
Exhibit 99.1

EyeGate Pharmaceuticals, Inc. Providing innovative products that enhance drug efficacy and patient compliance to improve vision Corporate Presentation

1 Forward Looking Statements Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s lead product EGP - 437, for the timing and outcome of the Company’s clinical trials, the potential approval to market EGP - 437, and the Company’s capital needs. Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation. Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful. The Company may consume more cash than it currently anticipates and faster than projected. Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates. If the U.S. Food and Drug Administration or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations. Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K filed with the SEC on February 23, 2017. The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law.

2 Company Overview Ophthalmology company (NASDAQ: EYEG) ▪ Platform 1: Crosslinked HA (eye drop formulation) • Corneal Epithelial Defects: • Positive results announced from pilot clinical trial • FDA De Novo 510(k) filing by year - end 2017 • European CE Mark by year - end 2017 ▪ Platform 2: Proprietary delivery system (delivering EGP - 437: corticosteroid) • Cataract Surgery: • Phase 2 trial to be initiated Q2 2017 • Supplemental NDA filing H2 2018 • Anterior Uveitis: • Second Phase 3 underway • NDA submission year - end 2017 Licensed to Valeant Pharmaceuticals (Bausch + Lomb)
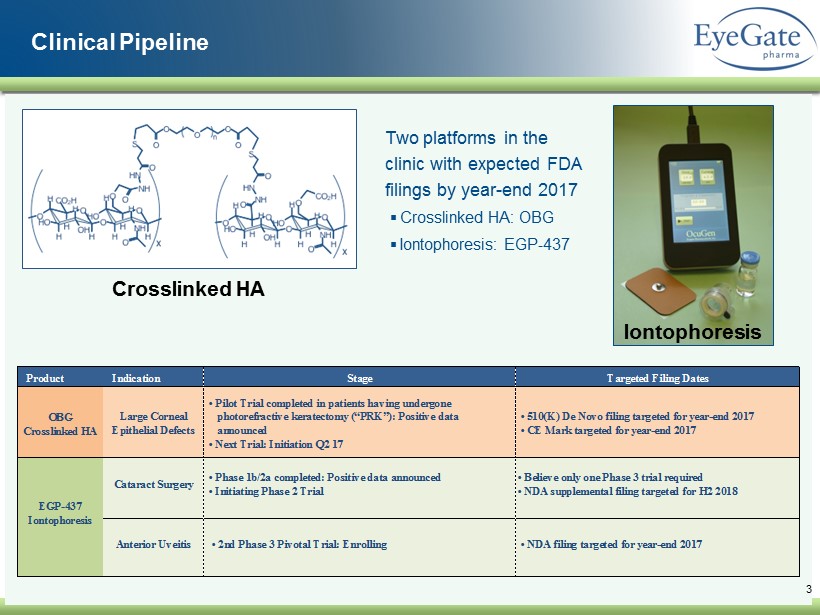
Clinical Pipeline 3 Iontophoresis Crosslinked HA Two platforms in the clinic with expected FDA filings by year - end 2017 ▪ Crosslinked HA: OBG ▪ Iontophoresis: EGP - 437 Product Indication Stage Targeted Filing Dates Large Corneal Epithelial Defects • Pilot Trial completed in patients having undergone photorefractive keratectomy (“PRK”): Positive data announced • Next Trial: Initiation Q2 17 • 510(K) De Novo filing targeted for year-end 2017 • CE Mark targeted for year-end 2017 OBG Crosslinked HA EGP-437 Iontophoresis Anterior Uveitis • 2nd Phase 3 Pivotal Trial: Enrolling • NDA filing targeted for year-end 2017 Cataract Surgery • Phase 1b/2a completed: Positive data announced • Initiating Phase 2 Trial • Believe only one Phase 3 trial required • NDA supplemental filing targeted for H2 2018

4 Hyaluronic Acid Hyaluronic acid is a naturally occurring compound in the body • ~15 grams of HA in an adult human body • About 50% in the skin (promotes wound healing), also in the synovial fluid (natural lubricant) • Rapidly degrades: one - third is naturally turned - over (degraded and synthesized) every day ▪ Properties • High - molecular weight HA is non - immunogenic • HA binds up to 1,000 times its volume in water • HA’s functions include: hydration, lubrication of joints, and providing a meshwork for cell migration HA approved for derm and osteoarthritis in the U.S. and for dry eye ex - U.S. • HA approved in U.S. as a dressing for wound and burn management (dermatology) • HA injections approved in the U.S. to treat knee pain caused by osteoarthritis • HA eye drops are the standard of care in Europe and Asia for symptoms and signs of dry eye and/or ocular surface damage, due to diseases such as superficial keratitis, Sjögren syndrome or primary dry eye syndrome and wound healing.
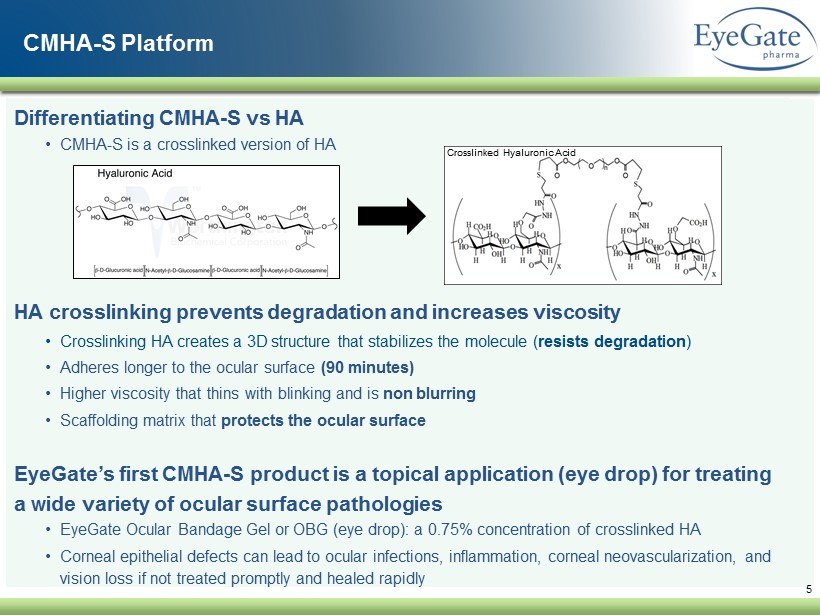
5 CMHA - S Platform Differentiating CMHA - S vs HA • CMHA - S is a crosslinked version of HA HA crosslinking prevents degradation and increases viscosity • Crosslinking HA creates a 3D structure that stabilizes the molecule ( resists degradation ) • Adheres longer to the ocular surface (90 minutes) • Higher viscosity that thins with blinking and is non blurring • Scaffolding matrix that protects the ocular surface EyeGate’s first CMHA - S product is a topical application (eye drop) for treating a wide variety of ocular surface pathologies • EyeGate Ocular Bandage Gel or OBG (eye drop): a 0.75% concentration of crosslinked HA • Corneal epithelial defects can lead to ocular infections, inflammation, corneal neovascularization, and vision loss if not treated promptly and healed rapidly Crosslinked Hyaluronic Acid
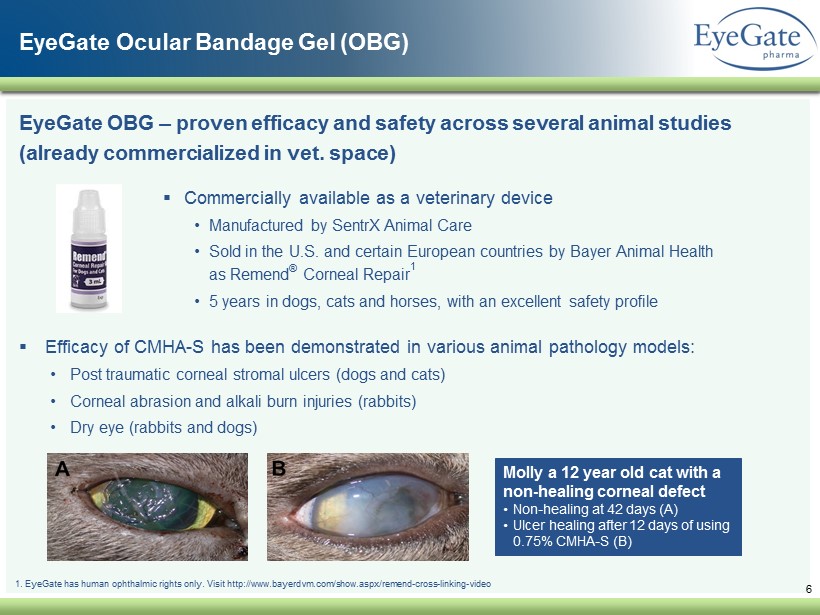
EyeGate OBG – proven efficacy and safety across several animal studies (already commercialized in vet. space) ▪ Efficacy of CMHA - S has been demonstrated in various animal pathology models: • Post traumatic corneal stromal ulcers (dogs and cats) • Corneal abrasion and alkali burn injuries (rabbits) • Dry eye (rabbits and dogs) 1. EyeGate has human ophthalmic rights only. Visit http://www.bayerdvm.com/show.aspx/remend - cross - linking - video ▪ Commercially available as a veterinary device • Manufactured by SentrX Animal Care • Sold in the U.S. and certain European countries by Bayer Animal Health as Remend ® Corneal Repair 1 • 5 years in dogs, cats and horses, with an excellent safety profile 6 Molly a 12 year old cat with a non - healing corneal defect • Non - healing at 42 days (A) • Ulcer healing after 12 days of using 0.75% CMHA - S (B) EyeGate Ocular Bandage Gel (OBG)
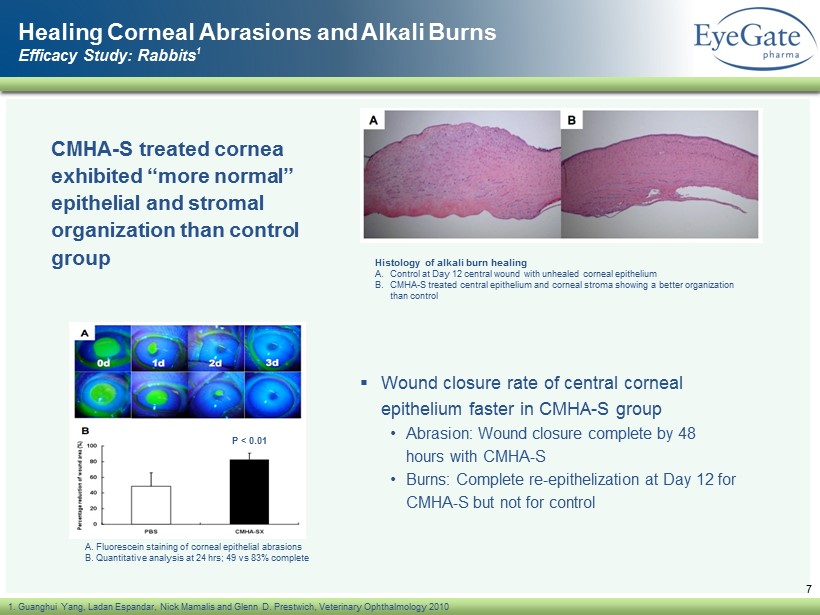
Healing Corneal Abrasions and Alkali Burns Efficacy Study: Rabbits 1 CMHA - S treated cornea exhibited “more normal” epithelial and stromal organization than control group 7 1. Guanghui Yang, Ladan Espandar , Nick Mamalis and Glenn D. Prestwich, Veterinary Ophthalmology 2010 A. Fluorescein staining of corneal epithelial abrasions B. Quantitative analysis at 24 hrs ; 49 vs 83% complete P < 0.01 Histology of alkali burn healing A. Control at Day 12 central wound with unhealed corneal epithelium B. CMHA - S treated central epithelium and corneal stroma showing a better organization than control ▪ Wound closure rate of central corneal epithelium faster in CMHA - S group • Abrasion: Wound closure complete by 48 hours with CMHA - S • Burns: Complete re - epithelization at Day 12 for CMHA - S but not for control
Announced positive data evaluating ability of EyeGate OBG to accelerate corneal surface re - epithelialization following bilateral photorefractive keratectomy (PRK) ▪ PRK is an efficacious alternative for patients seeking surgical correction of refractive errors who are poor LASIK candidates ▪ PRK surgery provides several advantages as indication to evaluate OBG’s ability • Larger epithelial defects : All eyes randomized at time zero with same size defect • Homogenous population: All eyes healthy (i.e. normal stem cell function) and will heal at ~ same rate ▪ 39 subjects randomized to one of 3 groups: both eyes received the same treatment • Group 1: EyeGate Ocular Bandage Gel QID for 2 weeks after surgery • Group 2: EyeGate Ocular Bandage Gel QID for 2 weeks after surgery in combination with a Bandage Contact Lens (BCL) • Group 3: BCL and preservative - free artificial tears 8 EyeGate Ocular Bandage Gel (OBG) First Human Clinical Trial Completed
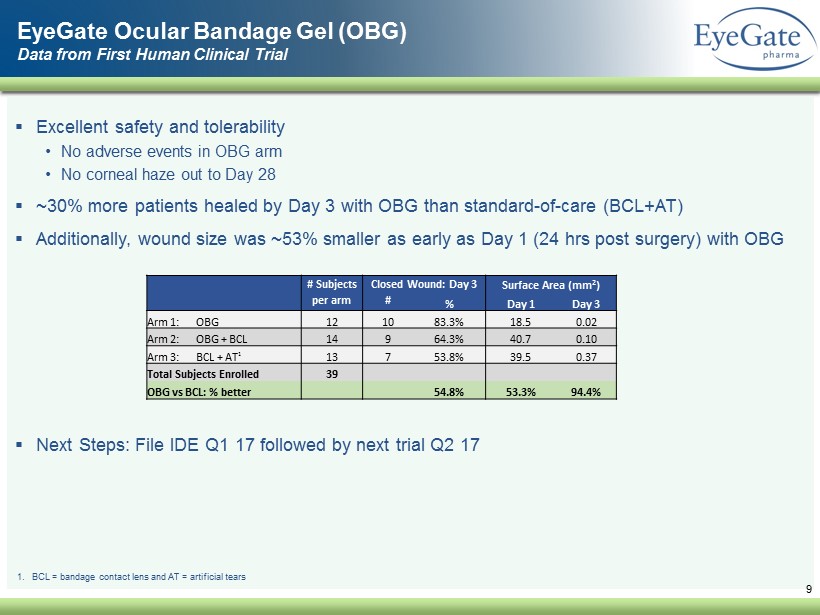
▪ Excellent safety and tolerability • No adverse events in OBG arm • No corneal haze out to Day 28 ▪ ~30% more patients healed by Day 3 with OBG than standard - of - care (BCL+AT) ▪ Additionally, wound size was ~53% smaller as early as Day 1 (24 hrs post surgery) with OBG ▪ Next Steps: File IDE Q1 17 followed by next trial Q2 17 9 EyeGate Ocular Bandage Gel (OBG) Data from First Human Clinical Trial 1. BCL = bandage contact lens and AT = artificial tears # Subjects Closed Wound: Day 3 Surface Area (mm 2 ) per arm # % Day 1 Day 3 Arm 1: OBG 12 10 83.3% 18.5 0.02 Arm 2: OBG + BCL 14 9 64.3% 40.7 0.10 Arm 3: BCL + AT 1 13 7 53.8% 39.5 0.37 Total Subjects Enrolled 39 OBG vs BCL: % better 54.8% 53.3% 94.4%

Meeting with FDA (Nov 2016) confirms device 510(k) de novo filing available for OBG Device - Indication for Use (IFU): Acceleration of re - epithelialization of large corneal epithelial defects in patients having undergone PRK ▪ Broader IFU: Demonstrate benefit in additional clinical trial(s) based on size of defect and not a specific underlying cause or indication • Superiority claim against standard - of - care not necessary 10 EyeGate Ocular Bandage Gel (OBG) Device CMHA - S ▪ Promotes re - epithelization (wound healing) ▪ Accelerates re - epithelization ▪ Exhibits “more normal” epithelial and stromal organization and morphology RESULT: POTENTIALLY FASTER RESTORATION OF VISION AND BETTER VISUAL OUTCOMES

EyeGate has retained control of worldwide commercial rights to CMHA - S • FDA 510(K) de novo filing targeted for year - end 2017 • European CE Mark targeted for year - end 2017, commercializing H1 2018 EyeGate Ocular Bandage Gel (OBG) Surgeries : >4 million (~ 0.8 million prescriptions) • R efractive surgeries ( PRK) • Vitrectomies (Diabetics) • Collagen crosslinking • P re - cataract surgery • Pterygium Ocular trauma: ~1.8 million (~0 .9 million prescriptions) • Corneal foreign bodies • A brasions / contusions • C hemical burns • D ifficult to heal alkali burns (PCED) Ocular/ Systemic Disorders: >50 million (~ 1.4 million prescriptions) • Neu rotrophic keratitis (Herpes, Diabetes) • Autoimmune disease ( Sjogren’s ) • GvHD • Contact lens wear • Ocular irritants Epithelial injury (exposure): ~16 million (~ 1.8 million prescriptions) • Lid malposition (ectropion/entropion) • Dry Eye • Cranial Neuropathies Corneal Epitheliopathy: U.S. Numbers 1 11 1. Potential peak sales based on EyeGate internal estimates, and are subject to unknown factors including, but not limited to, c lin ical outcome, regulatory approval, payer negotiations and competition. Potential peak sales amounts assume a price range of $150 to $250 per prescription and approximately 4.8 mi lli on prescriptions per year. See “Forward Looking Statements” above. These numbers include all people at risk for corneal epitheliopathies. OBG POTENTIAL PROJECTED U.S. PEAK SALES: BETWEEN $720 million AND $1.2 billion 1

12 CMHA - S Solid or Film Formulations (2 Versions) Research Funded by Grants ▪ Desired Properties of the Film: • Easy to place, requiring no sutures or glue • Allows for immediate stabilization of the eye following trauma • No refrigeration or freezer required: room stable • Prevents adhesions and scar formation between the globe and the conjunctiva 2. NSF SBIR Grant: Phase II Status ▪ Films/Pellet: Topical sustained - release delivery vehicle placed in inferior fornix • Release Profile: High - load product still releasing at 8 weeks ( in vitro study ongoing) • Retention Rate: Re - engineering design for longer retention on eye • Delivery vehicle for short or long - term acute or chronic conditions including • Antibiotic: bacterial conjunctivitis/keratitis • Antihistamine: seasonal/perennial allergies • Prostaglandins: glaucoma 1. DoD SBIR Phase II Grant: Ocular Surface Shield ▪ A sterile, field - stable product easily applied to immediately protect and promote healing of the ocular surface
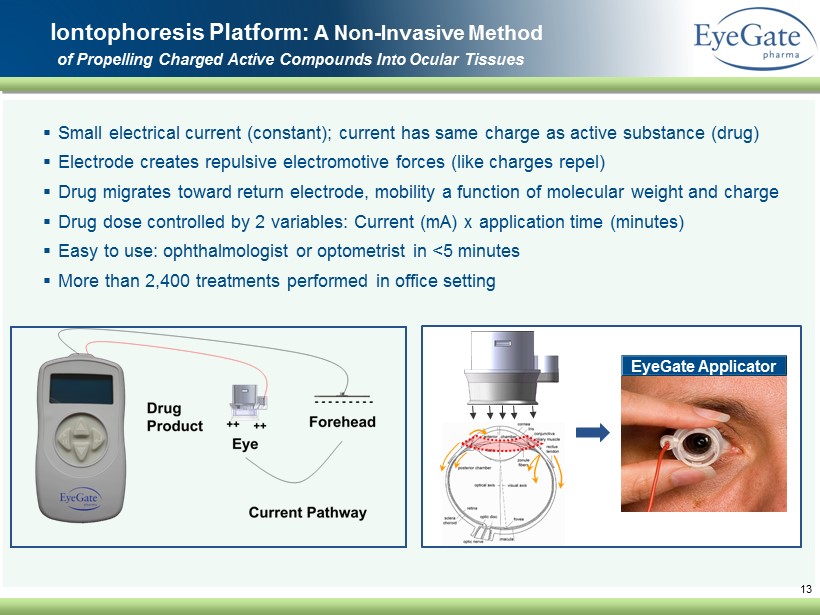
▪ Small electrical current (constant); current has same charge as active substance (drug) ▪ Electrode creates repulsive electromotive forces (like charges repel) ▪ Drug migrates toward return electrode, mobility a function of molecular weight and charge ▪ Drug dose controlled by 2 variables: Current (mA) x application time (minutes) ▪ Easy to use: ophthalmologist or optometrist in <5 minutes ▪ More than 2,400 treatments performed in office setting 13 Iontophoresis Platform: A Non - Invasive Method of Propelling Charged Active Compounds Into Ocular Tissues EyeGate Applicator
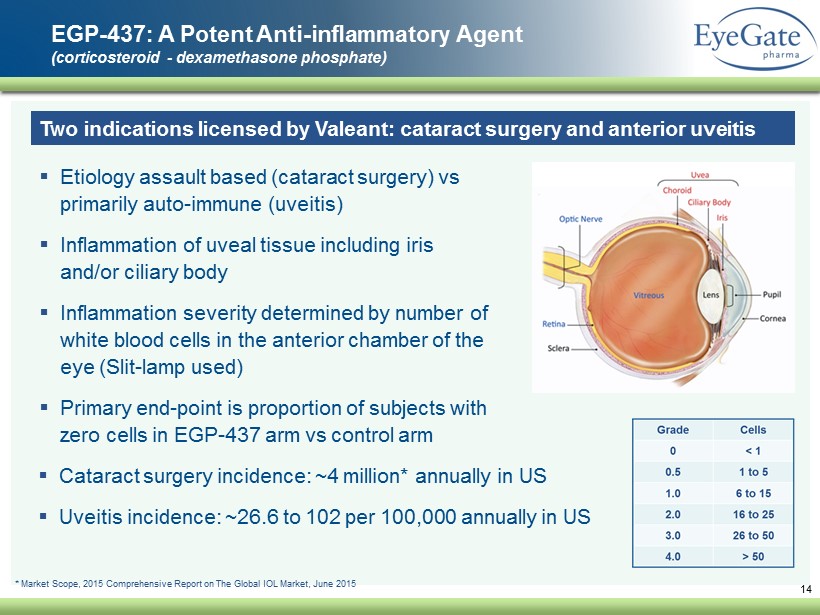
14 EGP - 437: A Potent Anti - inflammatory Agent (corticosteroid - dexamethasone phosphate) Two indications licensed by Valeant: cataract surgery and anterior uveitis ▪ Etiology assault based (cataract surgery) vs primarily auto - immune (uveitis) ▪ Inflammation of uveal tissue including iris and/or ciliary body ▪ Inflammation severity determined by number of white blood cells in the anterior chamber of the eye (Slit - lamp used) ▪ Primary end - point is proportion of subjects with zero cells in EGP - 437 arm vs control arm ▪ Cataract surgery incidence: ~4 million* annually in US ▪ Uveitis incidence: ~26.6 to 102 per 100,000 annually in US * Market Scope, 2015 Comprehensive Report on The Global IOL Market, June 2015
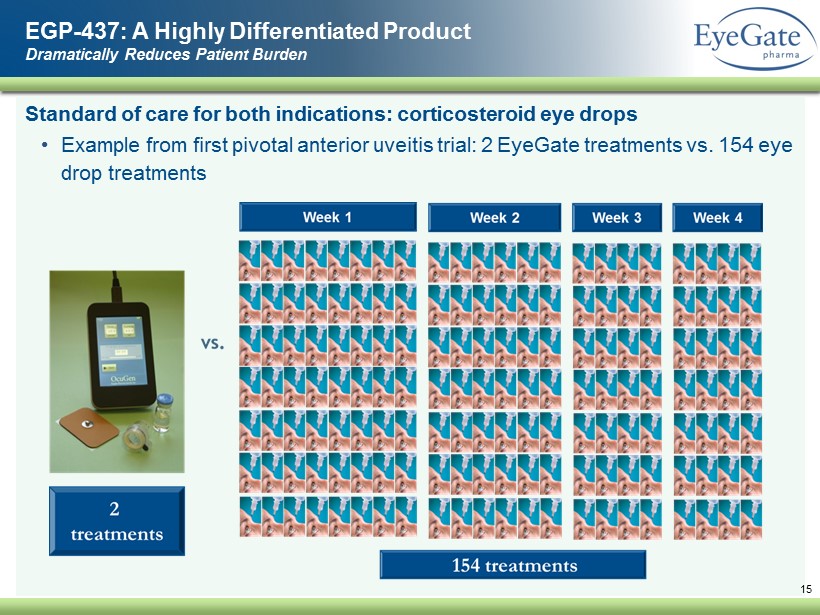
Standard of care for both indications: corticosteroid eye drops • Example from first pivotal anterior uveitis trial: 2 EyeGate treatments vs. 154 eye drop treatments EGP - 437: A Highly Differentiated Product Dramatically Reduces Patient Burden 15
16 Cataract Surgery ▪ EGP - 437 safe and effective in reducing inflammation and preventing pain as early as Day 1 with 2 different iontophoretic doses ▪ Trial design • 80 subjects who underwent unilateral cataract extraction with a monofocal intraocular lens • 7 cohorts whereby EGP - 437 was delivered in iontophoretic doses of 4.0 mA - min, 4.5 mA - min, 9.0 mA - min and 14.0 mA - min, 1 placebo cohort at 14.0 mA - min • Different dosing regimens: 2 or 3 doses, Day 0, Day 1, Day 4 and potential for additional treatment on Day 7 • Primary outcomes: • Proportion of subjects with anterior chamber cell (ACC) count of zero and • Proportion with pain score of zero ▪ Believe only one Phase 3 trial required: placebo controlled
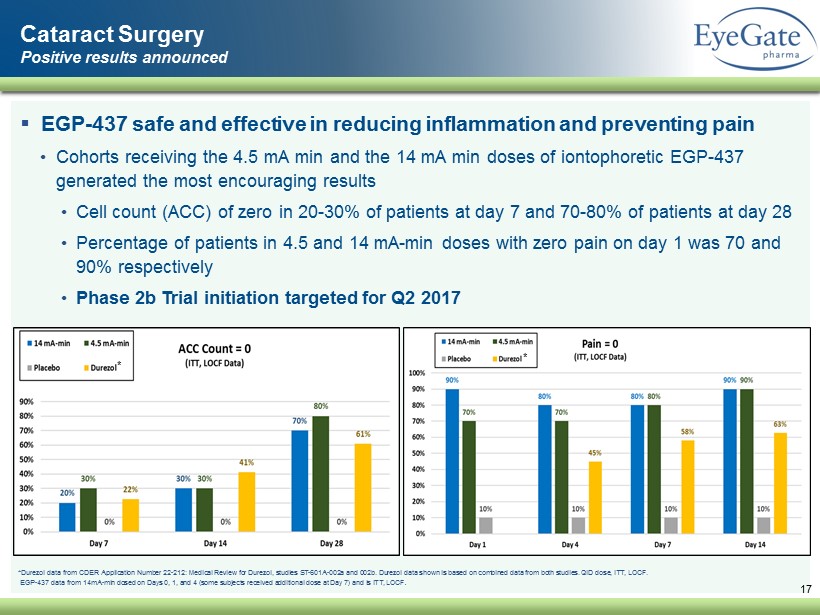
17 Cataract Surgery Positive results announced ▪ EGP - 437 safe and effective in reducing inflammation and preventing pain • Cohorts receiving the 4.5 mA min and the 14 mA min doses of iontophoretic EGP - 437 generated the most encouraging results • Cell count (ACC) of zero in 20 - 30% of patients at day 7 and 70 - 80% of patients at day 28 • Percentage of patients in 4.5 and 14 mA - min doses with zero pain on day 1 was 70 and 90% respectively • Phase 2b Trial initiation targeted for Q2 2017 * Durezol data from CDER Application Number 22 - 212: Medical Review for Durezol , studies ST - 601A - 002a and 002b. Durezol data shown is based on combined data from both studies. QID dose, ITT, LOCF. EGP - 437 data from 14mA - min dosed on Days 0, 1, and 4 (some subjects received additional dose at Day 7) and is ITT, LOCF . * *
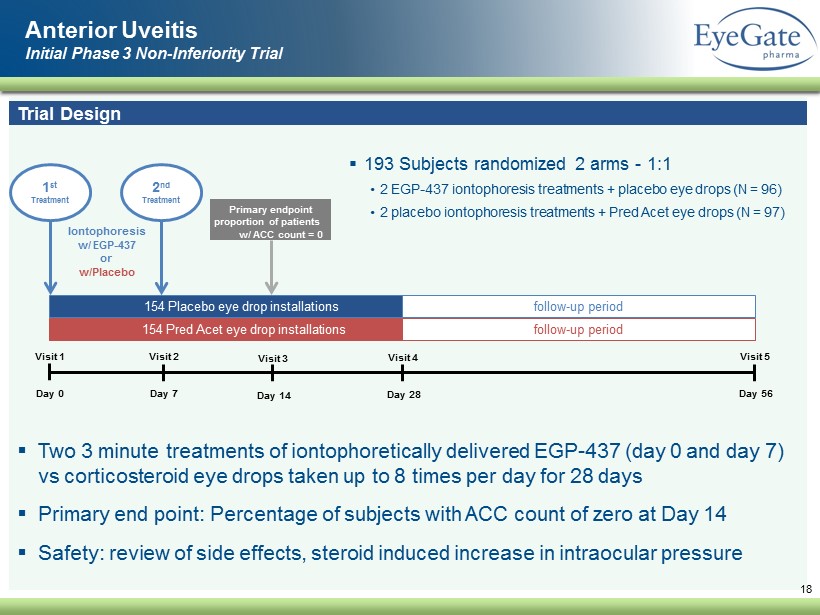
▪ Two 3 minute treatments of iontophoretically delivered EGP - 437 (day 0 and day 7) vs corticosteroid eye drops taken up to 8 times per day for 28 days ▪ Primary end point: Percentage of subjects with ACC count of zero at Day 14 ▪ Safety: review of side effects, steroid induced increase in intraocular pressure Anterior Uveitis Initial Phase 3 Non - Inferiority Trial 18 Trial Design ▪ 193 Subjects randomized 2 arms - 1:1 • 2 EGP - 437 iontophoresis treatments + placebo eye drops (N = 96) • 2 placebo iontophoresis treatments + Pred Acet eye drops (N = 97) Visit 1 Day 0 Visit 2 Day 7 Visit 3 Day 14 Visit 4 Day 28 Visit 5 Day 56 154 Pred Acet eye drop installations 154 Placebo eye drop installations follow - up period follow - up period 1 st Treatment Iontophoresis w/ EGP - 437 or w/Placebo Primary endpoint proportion of patients w/ ACC count = 0 2 nd Treatment
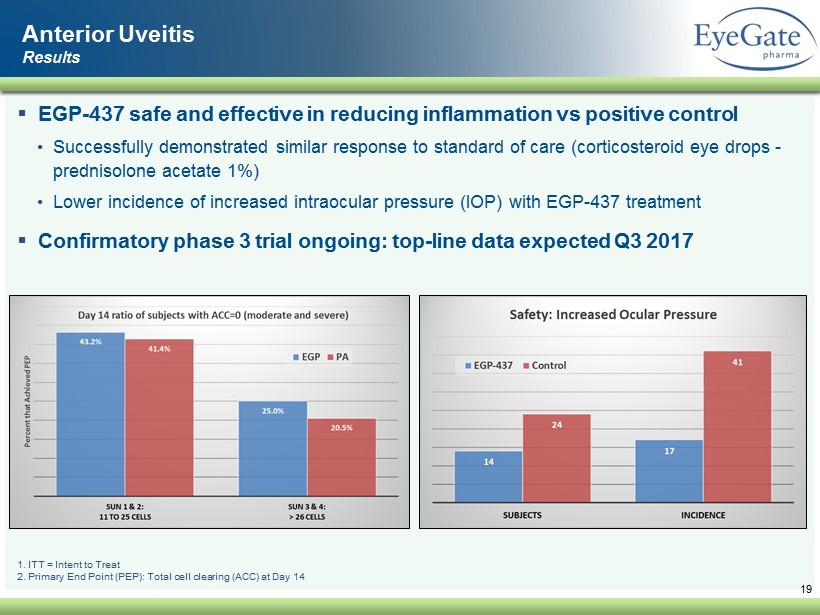
19 1. ITT = Intent to Treat 2. Primary End Point (PEP): Total cell clearing (ACC) at Day 14 ▪ EGP - 437 safe and effective in reducing inflammation vs positive control • Successfully demonstrated similar response to standard of care (corticosteroid eye drops - prednisolone acetate 1%) • Lower incidence of increased intraocular pressure (IOP) with EGP - 437 treatment ▪ Confirmatory phase 3 trial ongoing: top - line data expected Q3 2017 Anterior Uveitis Results

20 ▪ Valeant Pharmaceuticals – Bausch + Lomb (NYSE/TSX : VRX) ▪ Exclusive license to manufacture, sell, distribute and commercialize throughout the world for use in field of cataract surgery and uveitis • Total upfront and milestone payments of $ 135 million • Includes development milestones • Royalties based on net sales : high single digits with upward adjustment based on minimum sales for cataract surgery indication ▪ EyeGate responsible for completion of the clinical development and FDA filing for both indications ▪ Valeant responsible for development outside U . S . ▪ Valeant has right of last refusal for product outside of licensed fields • For EGP - 437 delivered with Iontophoretic EG II Delivery System Licensing Agreement EG® II Delivery System + EGP - 437
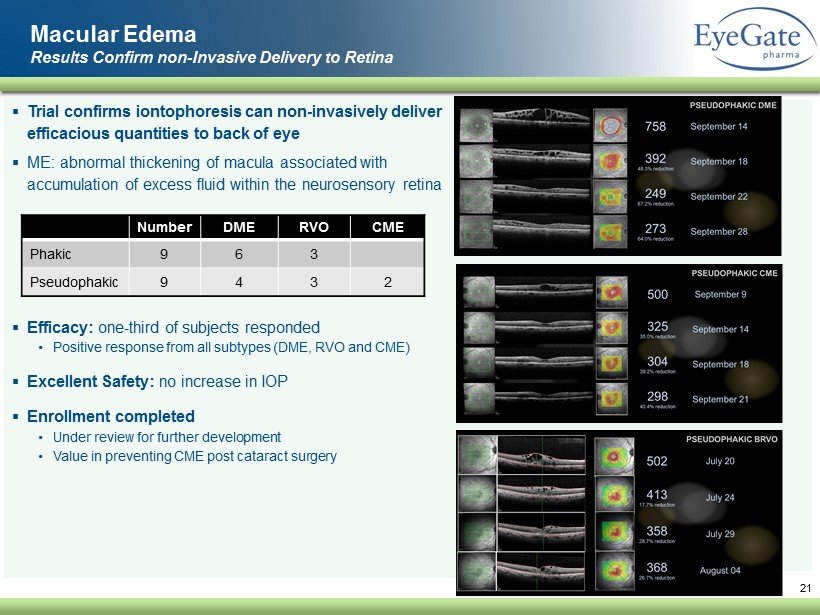
▪ Trial confirms iontophoresis can non - invasively deliver efficacious quantities to back of eye ▪ ME: abnormal thickening of macula associated with accumulation of excess fluid within the neurosensory retina ▪ Efficacy: one - third of subjects responded • Positive response from all subtypes (DME, RVO and CME) ▪ Excellent Safety: no increase in IOP ▪ Enrollment completed • Under review for further development • Value in preventing CME post cataract surgery 21 Macular Edema Results Confirm non - Invasive Delivery to Retina Number DME RVO CME Phakic 9 6 3 Pseudophakic 9 4 3 2
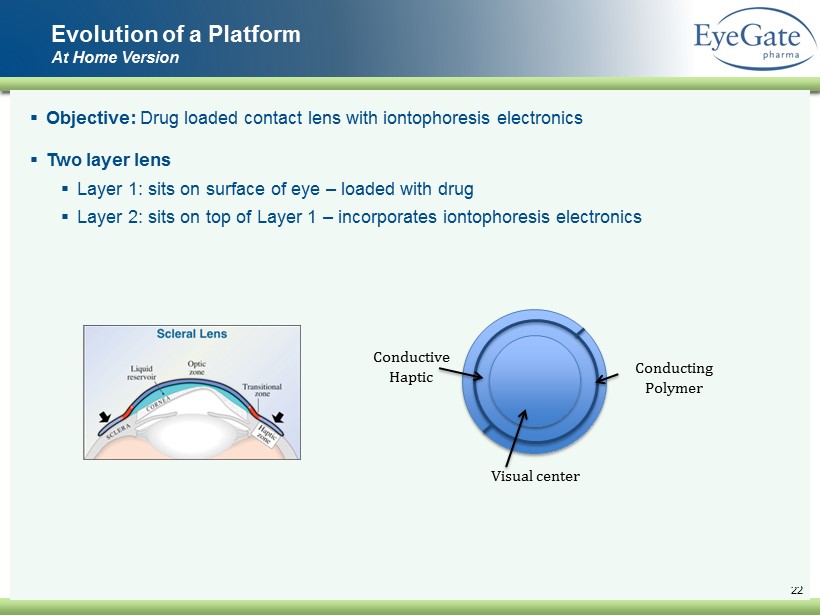
22 ▪ Objective: Drug loaded contact lens with iontophoresis electronics ▪ Two layer lens ▪ Layer 1: sits on surface of eye – loaded with drug ▪ Layer 2: sits on top of Layer 1 – incorporates iontophoresis electronics Evolution of a Platform At Home Version Visual center Conductive Haptic Conducting Polymer

23 Summary Ophthalmology company (NASDAQ: EYEG) ▪ OBG 510(K) de novo filing targeted for year - end 2017: first and only eye drop in the US with acceleration of re - epithelization claim ▪ OBG CE Mark targeted for year - end 2017, commercial launch Q1 2018 ▪ EGP - 437 NDA filing for Uveitis targeted for year - end 2017 ▪ EGP - 437 supplementary NDA filing for ocular surgery H2 2018: effectively controls post operative pain and inflammation without the need for drop therapy