EXHIBIT 99.1
Published on January 30, 2017
Exhibit 99.1

EyeGate Pharmaceuticals, Inc. Providing innovative products that enhance drug efficacy and patient compliance to improve vision Corporate Presentation

40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 1 Forward Looking Statements Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s lead product EGP - 437, for the timing and outcome of the Company’s clinical trials, the potential approval to market EGP - 437, and the Company’s capital needs. Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation. Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful. The Company may consume more cash than it currently anticipates and faster than projected. Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates. If the U.S. Food and Drug Administration or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations. Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K filed with the SEC on March 30, 2016 and the Company’s Quarterly Report on Form 10 - Q filed November 02, 2016. The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law.

40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 2 Company Overview ▪ Ophthalmology company (NASDAQ: EYEG) ▪ EGP - 437 (corticosteroid): proprietary delivery system • Anterior Uveitis: • NDA submission year - end 2017 • Licensed to Valeant Pharmaceuticals (Bausch + Lomb) • Cataract Surgery: • Phase 2 trial to be initiated Q1 2017 • Supplemental NDA filing H2 2018 ▪ Crosslinked HA (Eye drop formulation): • Photorefractive Keratectomy (PRK): • Positive results announced from first in man trial • FDA De Novo 510(k) filing by year - end 2017 • European CE Mark by year - end 2017
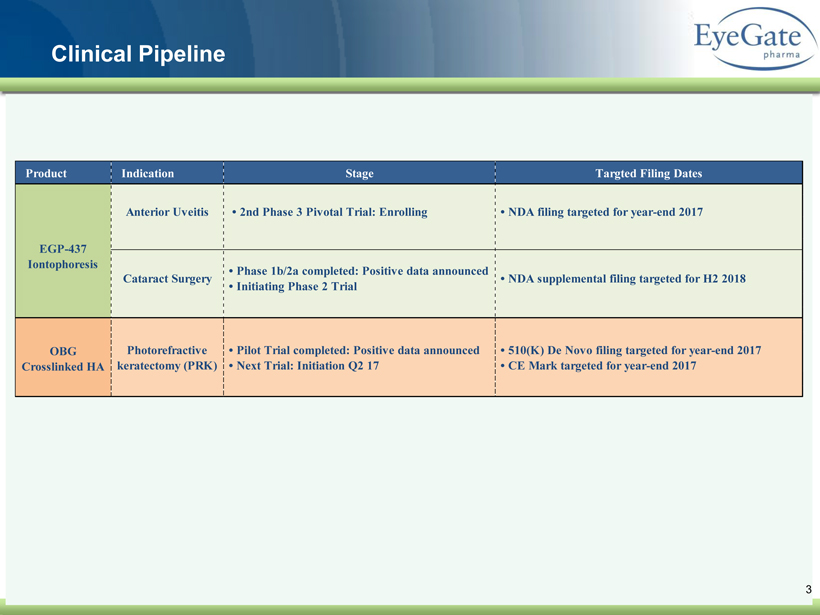
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 Clinical Pipeline 3 Product Indication Stage Targted Filing Dates Photorefractive keratectomy (PRK) OBG Crosslinked HA • Pilot Trial completed: Positive data announced • Next Trial: Initiation Q2 17 • 510(K) De Novo filing targeted for year-end 2017 • CE Mark targeted for year-end 2017 Anterior Uveitis • 2nd Phase 3 Pivotal Trial: Enrolling • NDA filing targeted for year-end 2017 EGP-437 Iontophoresis Cataract Surgery • Phase 1b/2a completed: Positive data announced • Initiating Phase 2 Trial • NDA supplemental filing targeted for H2 2018
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 4 Two Unique Ophthalmic Platforms Iontophoresis CMHA - S
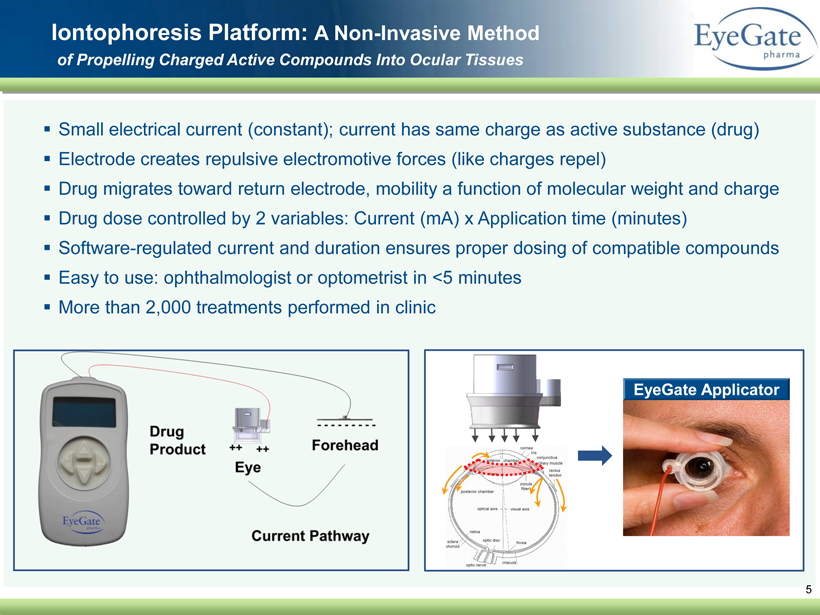
▪ Small electrical current (constant); current has same charge as active substance (drug) ▪ Electrode creates repulsive electromotive forces (like charges repel) ▪ Drug migrates toward return electrode, mobility a function of molecular weight and charge ▪ Drug dose controlled by 2 variables: Current (mA) x Application time (minutes) ▪ Software - regulated current and duration ensures proper dosing of compatible compounds ▪ Easy to use: ophthalmologist or optometrist in <5 minutes ▪ More than 2,000 treatments performed in clinic 5 Iontophoresis Platform: A Non - Invasive Method of Propelling Charged Active Compounds Into Ocular Tissues EyeGate Applicator
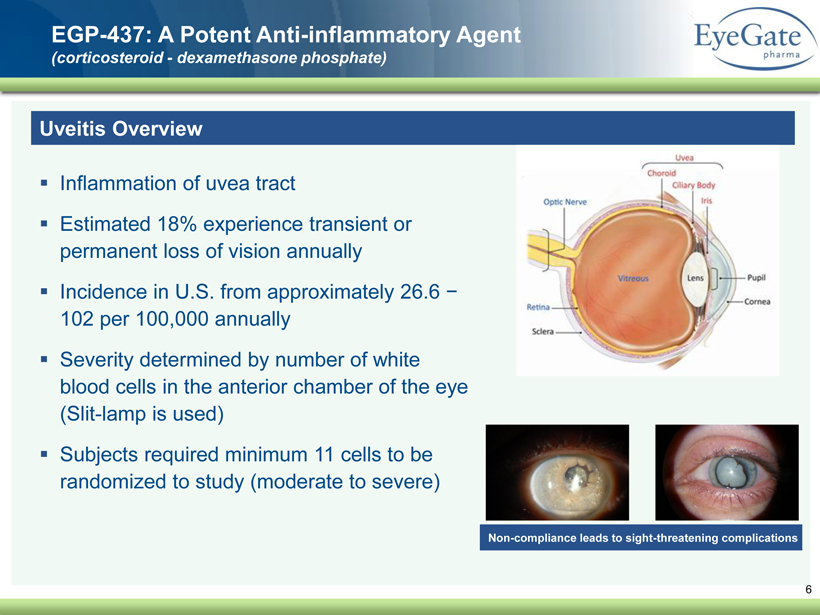
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 6 EGP - 437: A Potent Anti - inflammatory Agent (corticosteroid - dexamethasone phosphate) Uveitis Overview ▪ Inflammation of uvea tract ▪ Estimated 18% experience transient or permanent loss of vision annually ▪ Incidence in U.S. from approximately 26.6 − 102 per 100,000 annually ▪ Severity determined by number of white blood cells in the anterior chamber of the eye (Slit - lamp is used) ▪ Subjects required minimum 11 cells to be randomized to study (moderate to severe) Non - compliance leads to sight - threatening complications
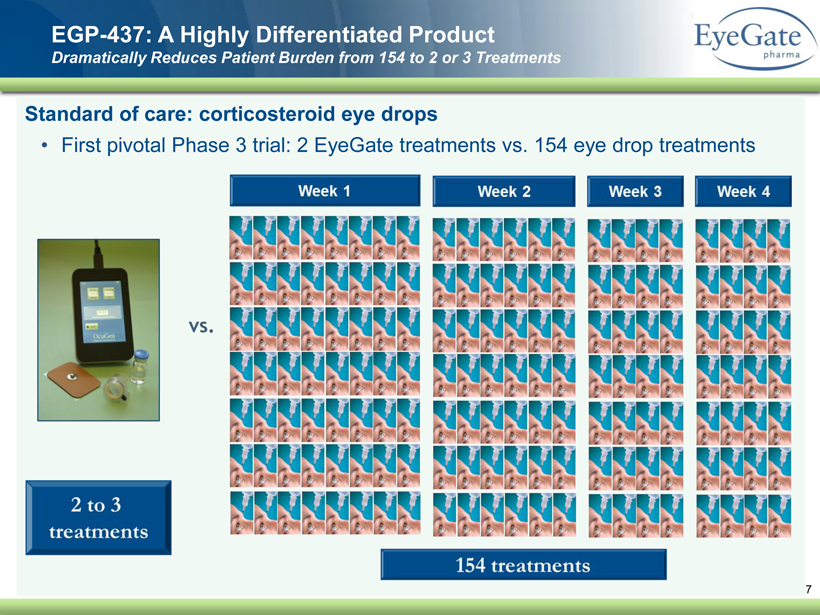
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 Standard of care: corticosteroid eye drops • First pivotal Phase 3 trial: 2 EyeGate treatments vs. 154 eye drop treatments EGP - 437: A Highly Differentiated Product Dramatically Reduces Patient Burden from 154 to 2 or 3 Treatments 7
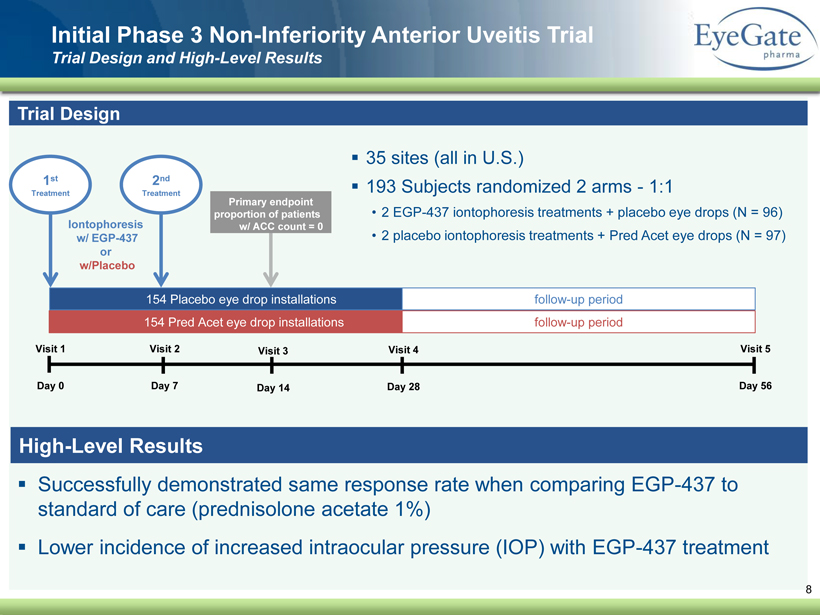
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 ▪ Successfully demonstrated same response rate when comparing EGP - 437 to standard of care (prednisolone acetate 1%) ▪ Lower incidence of increased intraocular pressure (IOP) with EGP - 437 treatment Initial Phase 3 Non - Inferiority Anterior Uveitis Trial Trial Design and High - Level Results 8 Trial Design ▪ 35 sites (all in U.S.) ▪ 193 Subjects randomized 2 arms - 1:1 • 2 EGP - 437 iontophoresis treatments + placebo eye drops (N = 96) • 2 placebo iontophoresis treatments + Pred Acet eye drops (N = 97) Visit 1 Day 0 Visit 2 Day 7 Visit 3 Day 14 Visit 4 Day 28 Visit 5 Day 56 154 Pred Acet eye drop installations 154 Placebo eye drop installations follow - up period follow - up period 1 st Treatment Iontophoresis w/ EGP - 437 or w/Placebo Primary endpoint proportion of patients w/ ACC count = 0 2 nd Treatment High - Level Results
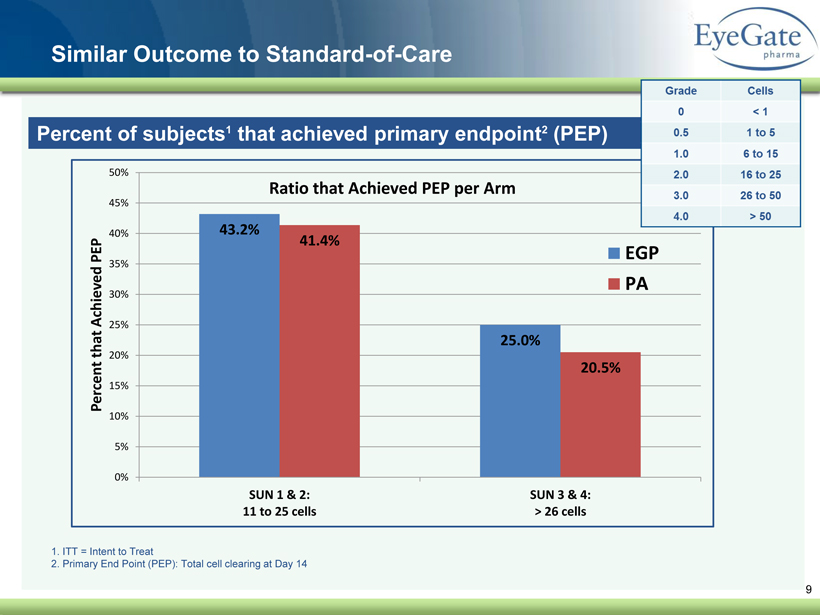
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 Similar Outcome to Standard - of - Care 9 Percent of subjects 1 that achieved primary endpoint 2 (PEP) 1. ITT = Intent to Treat 2. Primary End Point (PEP): Total cell clearing at Day 14 43.2% 25.0% 41.4% 20.5% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% SUN 1 & 2: 11 to 25 cells SUN 3 & 4: > 26 cells Percent that Achieved PEP Ratio that Achieved PEP per Arm EGP PA
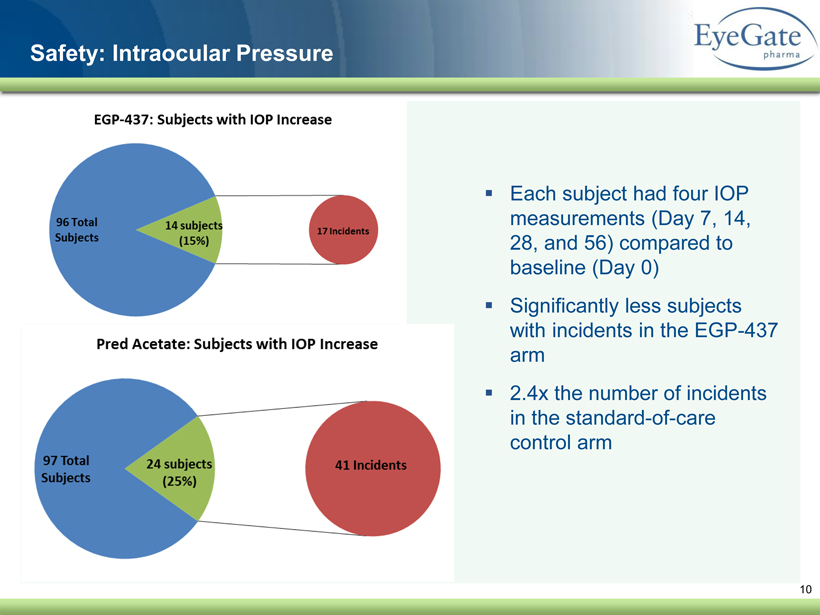
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 10 Safety: Intraocular Pressure ▪ Each subject had four IOP measurements (Day 7, 14, 28, and 56) compared to baseline (Day 0) ▪ Significantly less subjects with incidents in the EGP - 437 arm ▪ 2.4x the number of incidents in the standard - of - care control arm
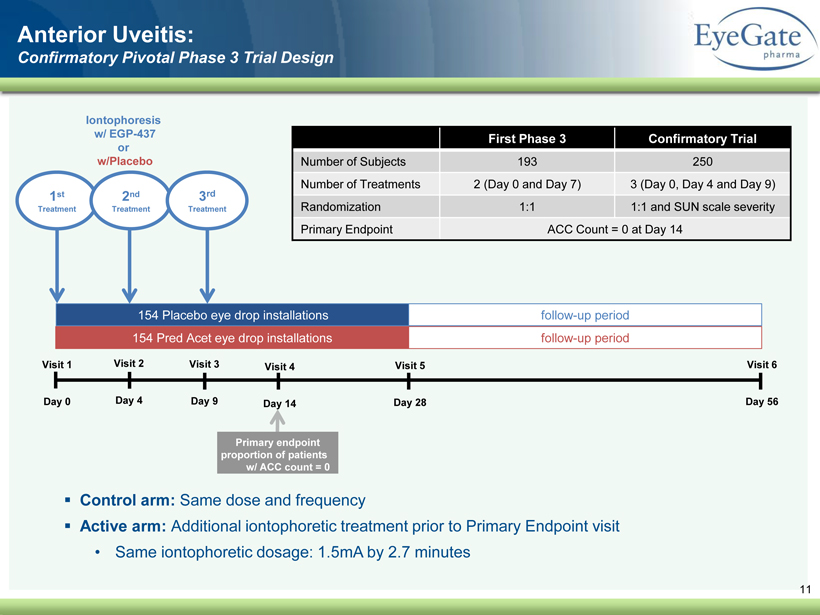
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 11 Visit 1 Day 0 Visit 3 Day 9 Visit 4 Day 14 Visit 5 Day 28 Visit 6 Day 56 154 Pred Acet eye drop installations 154 Placebo eye drop installations follow - up period follow - up period 1 st Treatment Iontophoresis w/ EGP - 437 or w/Placebo Primary endpoint proportion of patients w/ ACC count = 0 2 nd Treatment Anterior Uveitis: Confirmatory Pivotal Phase 3 Trial Design Visit 2 Day 4 3 rd Treatment ▪ Control arm: Same dose and frequency ▪ Active arm: Additional iontophoretic treatment prior to Primary Endpoint visit • Same iontophoretic dosage: 1.5mA by 2.7 minutes First Phase 3 Confirmatory Trial Number of Subjects 193 250 Number of Treatments 2 (Day 0 and Day 7) 3 (Day 0, Day 4 and Day 9) Randomization 1:1 1:1 and SUN scale severity Primary Endpoint ACC Count = 0 at Day 14

40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 12 ▪ Valeant Pharmaceuticals – Bausch + Lomb (NYSE/TSX : VRX) ▪ Exclusive license to manufacture, sell, distribute and commercialize throughout the world for use in field of uveitis • Upfront cash payment and milestone payments • Royalties based on net sales : high single digits ▪ EyeGate responsible for completion of the development of anterior uveitis indication in U . S . ▪ Valeant responsible for development outside U . S . ▪ Valeant has right of last refusal for product outside field of uveitis • Must negotiate for access to additional indications Licensing Agreement EG® II Delivery System + EGP - 437
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 13 EGP - 437: A Potent Anti - inflammatory Agent (corticosteroid - dexamethasone phosphate) Cataract Surgery Overview ▪ Ocular inflammation and pain are common side effects following cataract surgery • > 24 million people age 40 and older have cataracts in the US • Nearly four million cataract surgeries are performed each year in the US 1 ▪ Positive outcome from Phase 1b/2a, 80 subject open - label dose ranging trial • Subjects enrolled into cohorts (10 subjects/cohort) • Primary outcomes: • Proportion of subjects with anterior chamber cell (ACC) count of zero and • Proportion with pain score of zero 1. Market Scope, 2015 Comprehensive Report on The Global IOL Market, June 2015
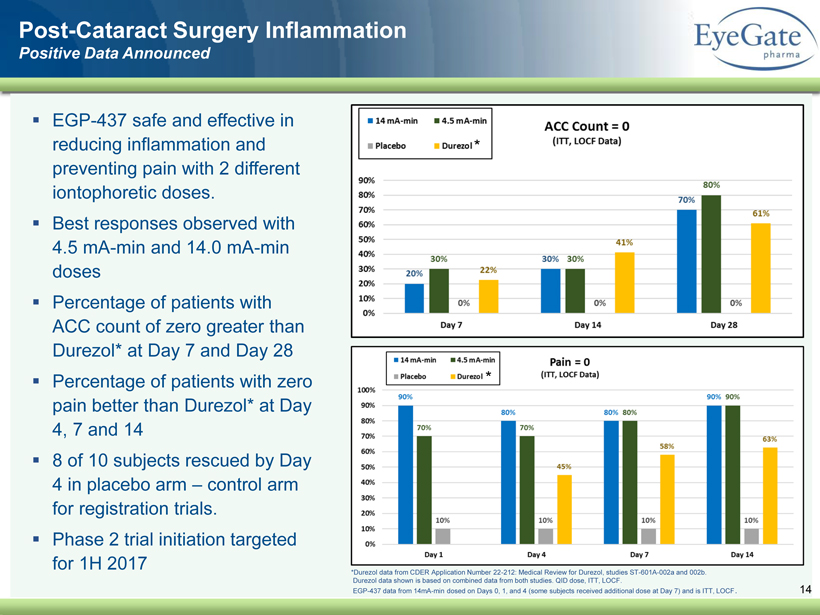
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 Post - Cataract Surgery Inflammation Positive Data Announced 14 * Durezol data from CDER Application Number 22 - 212: Medical Review for Durezol , studies ST - 601A - 002a and 002b. Durezol data shown is based on combined data from both studies. QID dose, ITT, LOCF. EGP - 437 data from 14mA - min dosed on Days 0, 1, and 4 (some subjects received additional dose at Day 7) and is ITT, LOCF . ▪ EGP - 437 safe and effective in reducing inflammation and preventing pain with 2 different iontophoretic doses. ▪ Best responses observed with 4.5 mA - min and 14.0 mA - min doses ▪ Percentage of patients with ACC count of zero greater than Durezol * at Day 7 and Day 28 ▪ Percentage of patients with zero pain better than Durezol * at Day 4, 7 and 14 ▪ 8 of 10 subjects rescued by Day 4 in placebo arm – control arm for registration trials. ▪ Phase 2 trial initiation targeted for 1H 2017 * *
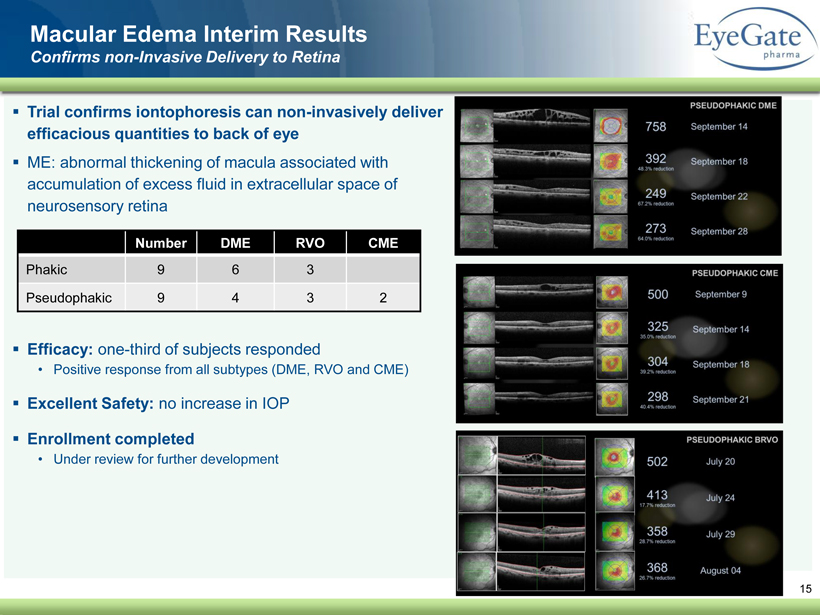
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 ▪ Trial confirms iontophoresis can non - invasively deliver efficacious quantities to back of eye ▪ ME: abnormal thickening of macula associated with accumulation of excess fluid in extracellular space of neurosensory retina ▪ Efficacy: one - third of subjects responded • Positive response from all subtypes (DME, RVO and CME) ▪ Excellent Safety: no increase in IOP ▪ Enrollment completed • Under review for further development 15 Macular Edema Interim Results Confirms non - Invasive Delivery to Retina Number DME RVO CME Phakic 9 6 3 Pseudophakic 9 4 3 2
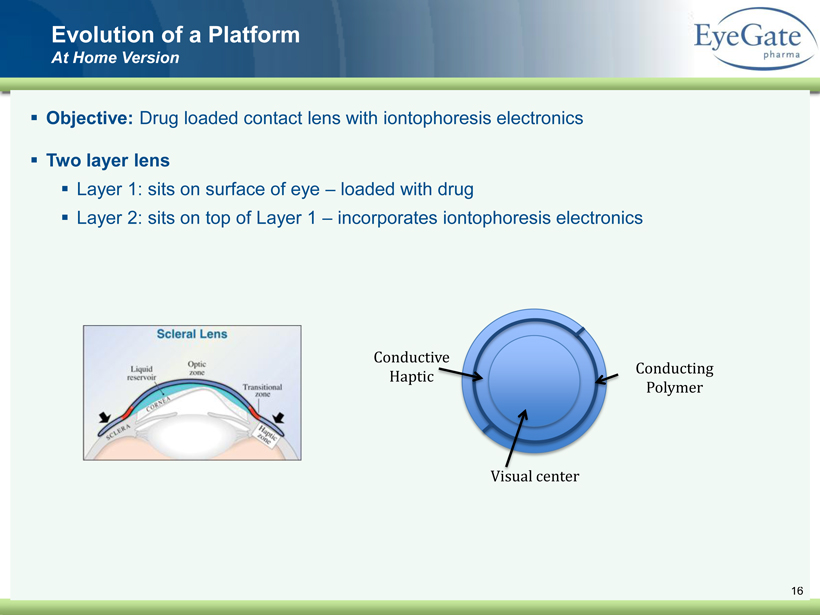
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 16 ▪ Objective: Drug loaded contact lens with iontophoresis electronics ▪ Two layer lens ▪ Layer 1: sits on surface of eye – loaded with drug ▪ Layer 2: sits on top of Layer 1 – incorporates iontophoresis electronics Evolution of a Platform At Home Version Visual center Conductive Haptic Conducting Polymer
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 17 Two Unique Ophthalmic Platforms Iontophoresis CMHA - S
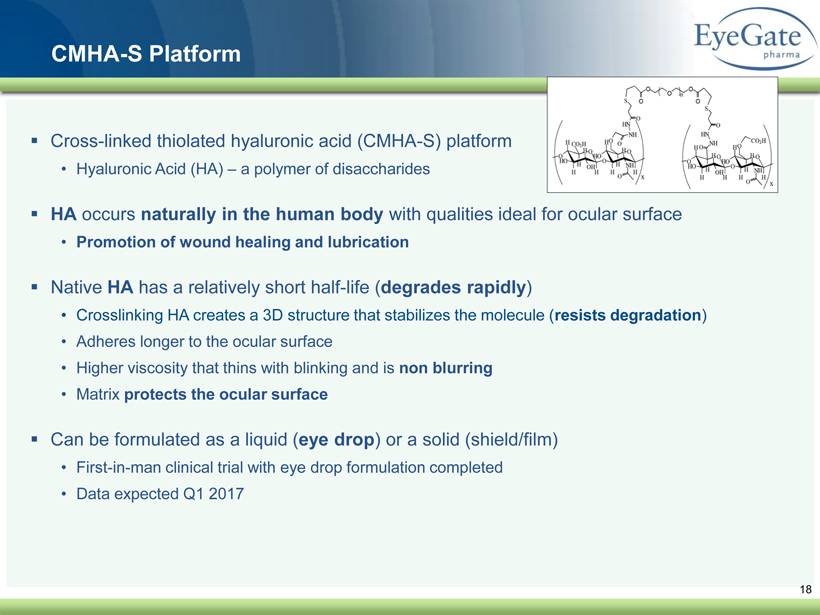
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 18 CMHA - S Platform ▪ Cross - linked thiolated hyaluronic acid (CMHA - S) platform • Hyaluronic Acid (HA) – a polymer of disaccharides ▪ HA occurs naturally in the human body with qualities ideal for ocular surface • Promotion of wound healing and lubrication ▪ Native HA has a relatively short half - life ( degrades rapidly ) • Crosslinking HA creates a 3D structure that stabilizes the molecule ( resists degradation ) • Adheres longer to the ocular surface • Higher viscosity that thins with blinking and is non blurring • Matrix protects the ocular surface ▪ Can be formulated as a liquid ( eye drop ) or a solid (shield/film) • First - in - man clinical trial with eye drop formulation completed • Data expected Q1 2017
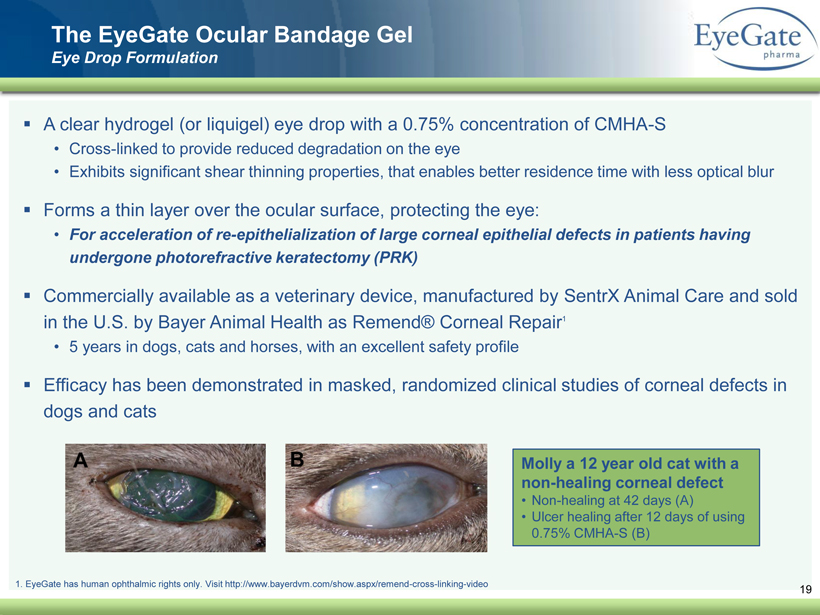
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 19 The EyeGate Ocular Bandage Gel Eye Drop Formulation ▪ A clear hydrogel (or liquigel ) eye drop with a 0.75% concentration of CMHA - S • Cross - linked to provide reduced degradation on the eye • Exhibits significant shear thinning properties, that enables better residence time with less optical blur ▪ Forms a thin layer over the ocular surface, protecting the eye: • For acceleration of re - epithelialization of large corneal epithelial defects in patients having undergone photorefractive keratectomy (PRK) ▪ Commercially available as a veterinary device, manufactured by SentrX Animal Care and sold in the U.S. by Bayer Animal Health as Remend ® Corneal Repair 1 • 5 years in dogs, cats and horses, with an excellent safety profile ▪ Efficacy has been demonstrated in masked, randomized clinical studies of corneal defects in dogs and cats Molly a 12 year old cat with a non - healing corneal defect • Non - healing at 42 days (A) • Ulcer healing after 12 days of using 0.75% CMHA - S (B) 1. EyeGate has human ophthalmic rights only. Visit http://www.bayerdvm.com/show.aspx/remend - cross - linking - video
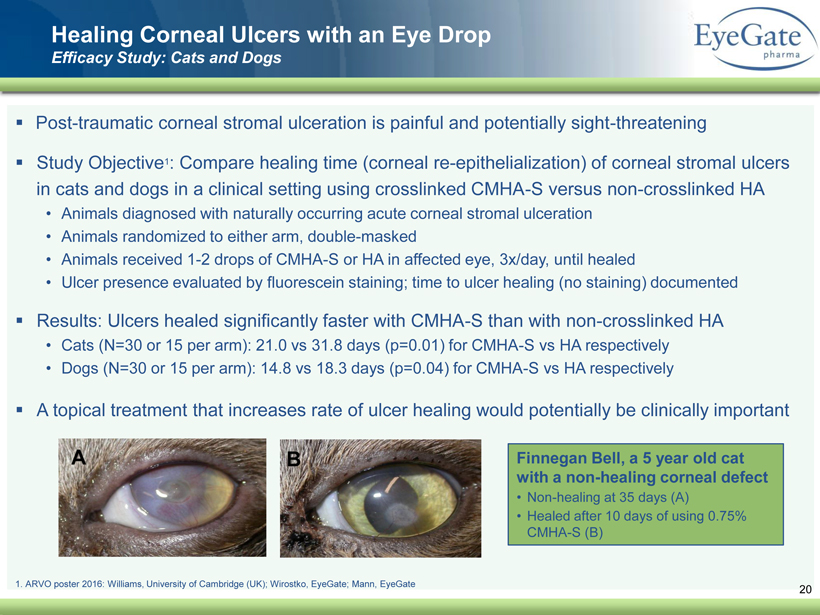
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 Healing Corneal Ulcers with an Eye Drop Efficacy Study: Cats and Dogs ▪ Post - traumatic corneal stromal ulceration is painful and potentially sight - threatening ▪ Study Objective 1 : Compare healing time (corneal re - epithelialization) of corneal stromal ulcers in cats and dogs in a clinical setting using crosslinked CMHA - S versus non - crosslinked HA • Animals diagnosed with naturally occurring acute corneal stromal ulceration • Animals randomized to either arm, double - masked • Animals received 1 - 2 drops of CMHA - S or HA in affected eye, 3x/day, until healed • Ulcer presence evaluated by fluorescein staining; time to ulcer healing (no staining) documented ▪ Results: Ulcers healed significantly faster with CMHA - S than with non - crosslinked HA • Cats (N=30 or 15 per arm): 21.0 vs 31.8 days (p=0.01) for CMHA - S vs HA respectively • Dogs (N=30 or 15 per arm): 14.8 vs 18.3 days (p=0.04) for CMHA - S vs HA respectively ▪ A topical treatment that increases rate of ulcer healing would potentially be clinically important Finnegan Bell, a 5 year old cat with a non - healing corneal defect • Non - healing at 35 days (A) • Healed after 10 days of using 0.75% CMHA - S (B) 20 1. ARVO poster 2016: Williams, University of Cambridge (UK); Wirostko, EyeGate ; Mann, EyeGate
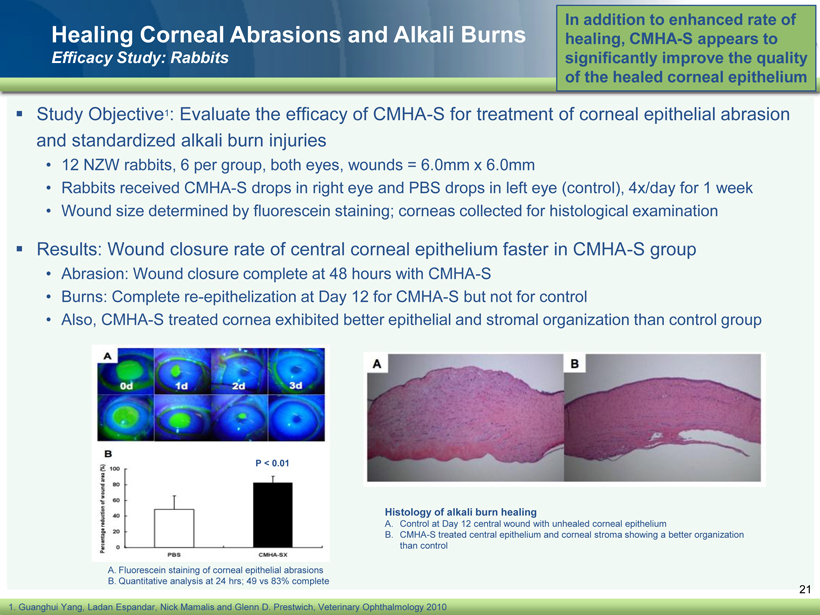
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 Healing Corneal Abrasions and Alkali Burns Efficacy Study: Rabbits ▪ Study Objective 1 : Evaluate the efficacy of CMHA - S for treatment of corneal epithelial abrasion and standardized alkali burn injuries • 12 NZW rabbits, 6 per group, both eyes, wounds = 6.0mm x 6.0mm • Rabbits received CMHA - S drops in right eye and PBS drops in left eye (control), 4x/day for 1 week • Wound size determined by fluorescein staining; corneas collected for histological examination ▪ Results: Wound closure rate of central corneal epithelium faster in CMHA - S group • Abrasion: Wound closure complete at 48 hours with CMHA - S • Burns: Complete re - epithelization at Day 12 for CMHA - S but not for control • Also, CMHA - S treated cornea exhibited better epithelial and stromal organization than control group In addition to enhanced rate of healing, CMHA - S appears to significantly improve the quality of the healed corneal epithelium 21 1. Guanghui Yang, Ladan Espandar , Nick Mamalis and Glenn D. Prestwich, Veterinary Ophthalmology 2010 A. Fluorescein staining of corneal epithelial abrasions B. Quantitative analysis at 24 hrs ; 49 vs 83% complete P < 0.01 Histology of alkali burn healing A. Control at Day 12 central wound with unhealed corneal epithelium B. CMHA - S treated central epithelium and corneal stroma showing a better organization than control

40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 22 ▪ Epithelial defects can lead to ocular infections, inflammation, corneal neovascularization, and vision loss if not treated promptly and healed rapidly. • Infectious keratitis (corneal infections and ulcers) that result from an exposed corneal surface can be a major cause of vision loss. ▪ The World Health Organization estimates that corneal opacities, including corneal ulceration, are the 4 th leading cause of blindness in the world. • Annual occurrence of corneal ulcers is roughly 1.5 to 2 million cases ▪ Billion $ opportunity as corneal epithelial defects are highly prevalent • 18% of emergency room visits (trauma, work related injuries) • Military (chemical and blast injuries) • Diabetics with corneal wounds • Superficial Punctate Keratitis • Surgical procedures including, Lasik, Photorefractive Keratectomy (PRK) and Cataract ▪ NO eye drop approved or available in the US for accelerating corneal re - epithelization Large Underserved Market The EyeGate Ocular Bandage Gel
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 ▪ Initial pilot study will be for photorefractive keratectomy (PRK) patients • Objective is to evaluate safety and performance of our eye drop administered QID for 14 days with or without a bandage contact lens (BCL) as compared to artificial tears and a bandage contact lens. • Primary effectiveness endpoint will be time to re - epithelization of epithelial defect following PRK surgery • Prospective, randomized, controlled study in up to 39 subjects undergoing bilateral PRK surgery ▪ Subjects will be randomized 1:1:1 to one of 3 groups, with both eyes of each subject receiving the same treatment: • Arm 1: EyeGate Ocular Bandage Gel QID for 2 weeks after surgery without a BCL • Arm 2: EyeGate Ocular Bandage Gel QID for 2 weeks after surgery in combination with a BCL • Arm 3: Artificial tears and BCL ▪ PRK is an efficacious alternative for patients seeking surgical correction of refractive errors who are not suitable candidates for LASIK. • The military prefers PRK surgery due to the stability of the PRK incision and the absence of risk for flap dislocation during military active duty . ▪ Expect top - line data prior to year - end 2016 • Intended for management of corneal epithelial defects and for the acceleration of re - epithelization of the ocular surface following surgery, injection, traumatic, and non - traumatic conditions. 23 The EyeGate Ocular Bandage Gel Clinical Trial Design
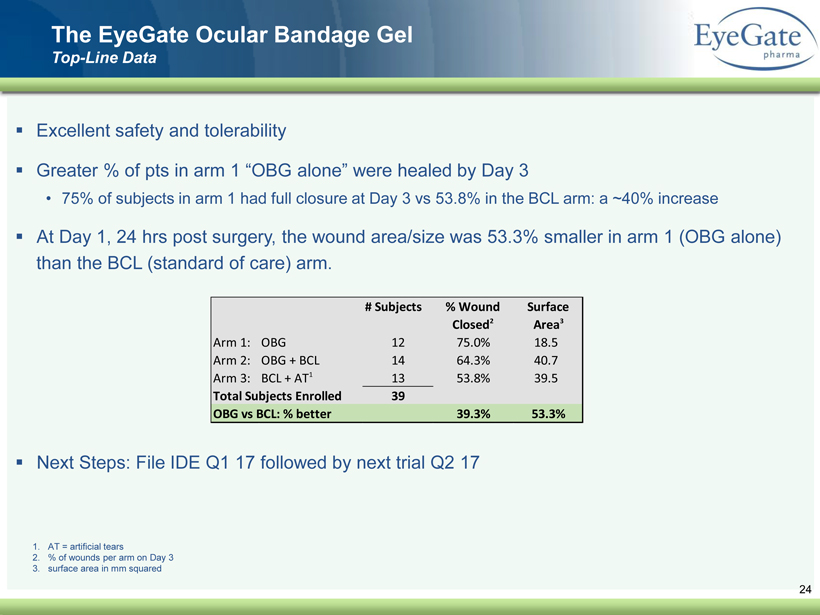
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 ▪ Excellent safety and tolerability ▪ Greater % of pts in arm 1 “OBG alone” were healed by Day 3 • 75% of subjects in arm 1 had full closure at Day 3 vs 53.8% in the BCL arm: a ~40% increase ▪ At Day 1, 24 hrs post surgery, the wound area/size was 53.3% smaller in arm 1 (OBG alone) than the BCL (standard of care) arm. ▪ Next Steps: File IDE Q1 17 followed by next trial Q2 17 24 The EyeGate Ocular Bandage Gel Top - Line Data # Subjects % Wound Closed 2 Surface Area 3 Arm 1: OBG 12 75.0% 18.5 Arm 2: OBG + BCL 14 64.3% 40.7 Arm 3: BCL + AT 1 13 53.8% 39.5 Total Subjects Enrolled 39 OBG vs BCL: % better 39.3% 53.3% 1. AT = artificial tears 2. % of wounds per arm on Day 3 3. surface area in mm squared
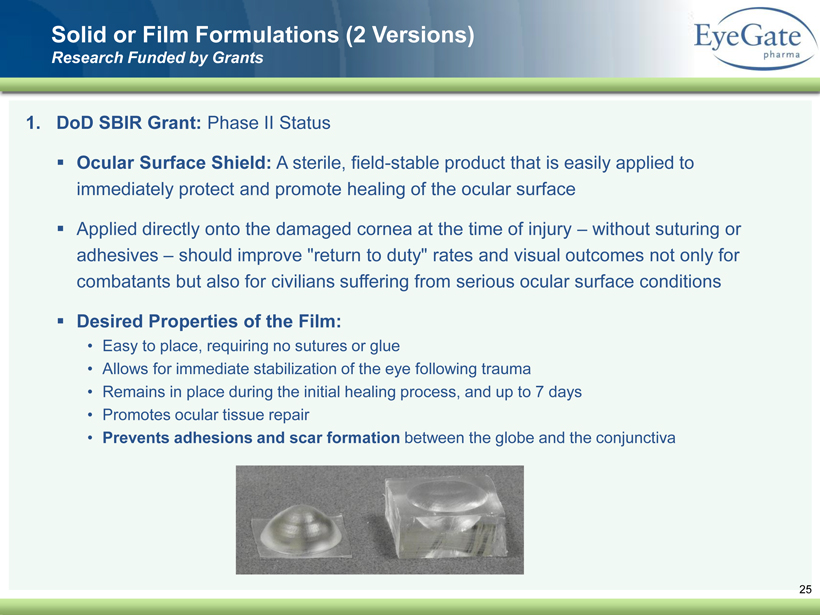
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 25 Solid or Film Formulations (2 Versions) Research Funded by Grants 1. DoD SBIR Grant: Phase II Status ▪ Ocular Surface Shield: A sterile, field - stable product that is easily applied to immediately protect and promote healing of the ocular surface ▪ Applied directly onto the damaged cornea at the time of injury – without suturing or adhesives – should improve "return to duty" rates and visual outcomes not only for combatants but also for civilians suffering from serious ocular surface conditions ▪ Desired Properties of the Film: • Easy to place, requiring no sutures or glue • Allows for immediate stabilization of the eye following trauma • Remains in place during the initial healing process, and up to 7 days • Promotes ocular tissue repair • Prevents adhesions and scar formation between the globe and the conjunctiva
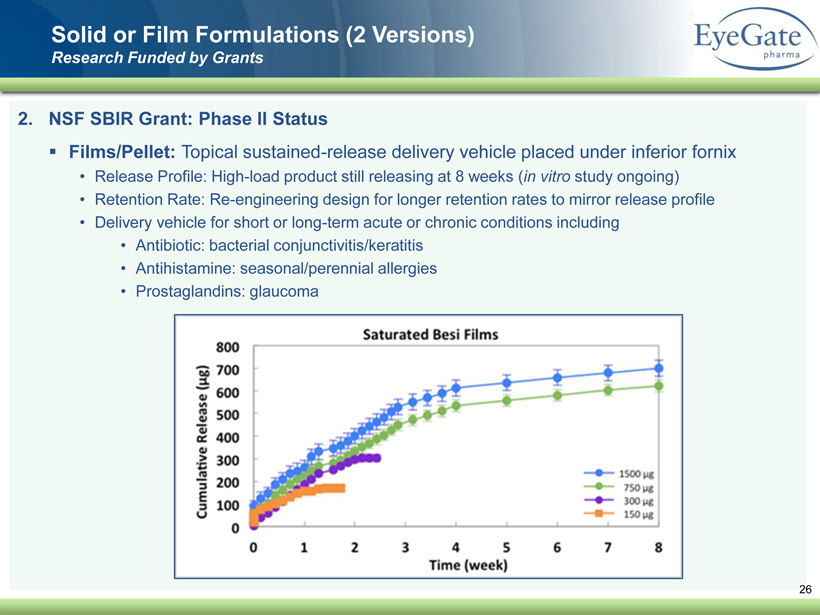
40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 2. NSF SBIR Grant: Phase II Status ▪ Films/Pellet: Topical sustained - release delivery vehicle placed under inferior fornix • Release Profile: High - load product still releasing at 8 weeks ( in vitro study ongoing) • Retention Rate: Re - engineering design for longer retention rates to mirror release profile • Delivery vehicle for short or long - term acute or chronic conditions including • Antibiotic: bacterial conjunctivitis/keratitis • Antihistamine: seasonal/perennial allergies • Prostaglandins: glaucoma Solid or Film Formulations (2 Versions) Research Funded by Grants 26

40 82 140 147 189 107 177 194 217 109 141 72 254 215 84 176 216 190 142 163 194 241 249 244 191 201 221 25 52 87 27 Company Overview ▪ Ophthalmology company (NASDAQ: EYEG) ▪ EGP - 437 (corticosteroid): proprietary delivery system • Anterior Uveitis: • NDA submission year - end 2017 • Licensed to Valeant Pharmaceuticals (Bausch + Lomb) • Cataract Surgery: • Phase 2 trial to be initiated Q1 2017 • Supplemental NDA filing H2 2018 ▪ Crosslinked HA (Eye drop formulation): • Photorefractive Keratectomy (PRK): • Positive results announced from first in man trial • FDA De Novo 510(k) filing by year - end 2017 • European CE Mark by year - end 2017