EXHIBIT 99.1
Published on March 7, 2016
Exhibit 99.1

EyeGate Pharmaceuticals, Inc. Providing innovative products that enhance drug efficacy and patient compliance to improve vision Acquisition of Jade Therapeutics

1 Forward Looking Statements Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s lead product EGP - 437, for the timing and outcome of the Company’s clinical trials, the potential approval to market EGP - 437, and the Company’s capital needs. Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation. Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful. The Company may consume more cash than it currently anticipates and faster than projected. Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates. If the FDA or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations. Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K filed with the SEC on March 31, 2015. The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law.

2 Acquisition Overview ▪ Consideration ▪ Will repay Jade liabilities of up to $ 300,000 ▪ Will issue 765,728 shares of EyeGate common stock (10% of outstanding common stock) • 90 % were issued at closing and 10% held back for 18 months to satisfy post - closing adjustments or indemnification obligations . ▪ Also includes a cash earn - out provision with an additional payment of up to $ 2,164,451, contingent upon a Jade product receiving FDA marketing approval ▪ Cash ▪ Grants: 2 ongoing grants from NSF and DoD with ~ $1.14 million available over the next 18 months ▪ Absorption Systems: ~ $370k credit available for preclinical work

3 Acquisition Overview ▪ Corporate ▪ EYEG will have R&D facility in Salt Lake City, Utah, home of Jade ▪ Strong research team ▪ Senior management has joined EYEG Barbara Wirostko M.D. Chief Medical Officer • A co - founder of Jade Therapeutics and a board certified ophthalmologist • Maintains academic research and clinical practice with the University of Utah, Moran Eye Center, as a Clinical Adjunct Associate Professor in Ophthalmology and as an Adjunct Associate Professor of Bioengineering • Former Development Lead and Senior Medical Director at Pfizer, where she led a successful EU regulatory EMA filing for Xalatan in pediatric glaucoma Brenda Mann, Ph.D. VP R&D • Has extensive experience with hydrogels for wound healing and drug delivery applications • A co - founder and VP for R&D at SentrX Animal Care, SLC, Utah focused on veterinary biomaterials • Adjunct Associate Professor in the Department of Bioengineering at the University of Utah • A founding faculty member of the Keck Graduate Institute of Applied Life Sciences, and now serves on its Advisory Council
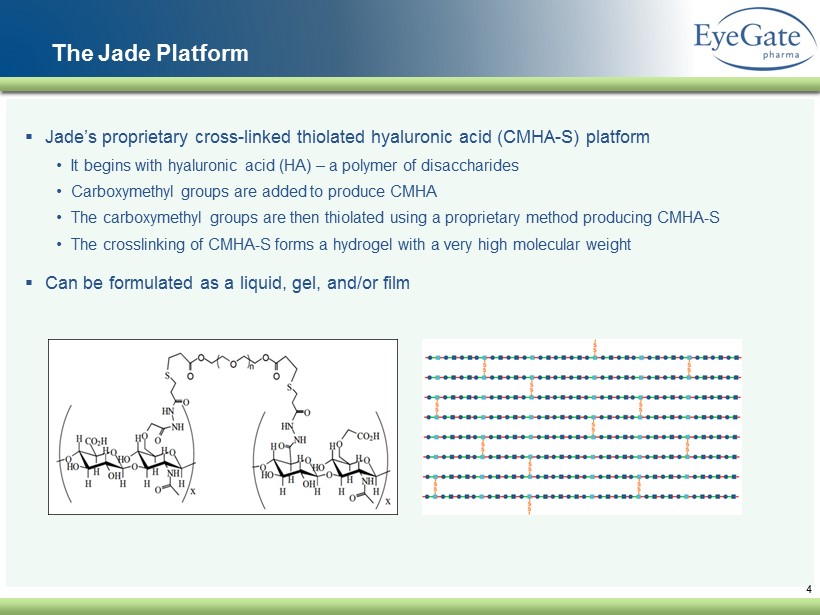
4 The Jade Platform ▪ Jade’s proprietary cross - linked thiolated hyaluronic acid (CMHA - S) platform • It begins with hyaluronic acid ( HA) – a polymer of disaccharides • Carboxymethyl groups are added to produce CMHA • The carboxymethyl groups are then thiolated using a proprietary method producing CMHA - S • The crosslinking of CMHA - S forms a hydrogel with a very high molecular weight ▪ Can be formulated as a liquid , gel, and/or film
5 Why Hyaluronic Acid (HA) ▪ It occurs naturally in the human body and is central to regulating cell growth and renewal • Has intrinsic wound - healing capabilities, as well as anti - inflammatory and anti - adhesive properties ▪ HA is found extensively in connective, epithelial, and neural cells • A major component of skin, where it is involved in tissue repair. • A major component of the synovial fluid and is one of the fluid's main lubricating components. ▪ HA binds to 3 main cell surface receptors: CD44, Receptor for HA - mediated motility (RHAMM) and intercellular adhesion molecule - 1 (ICAM - 1) • CD44 plays a part in various physiologic events such as cell aggregation, migration, proliferation and activation • ICAM - 1 binding may contribute to the control of ICAM - 1 - mediated inflammatory activation ▪ Since HA plays a key role in tissue repair it has applications in several medical treatments • U sed extensively to speed healing of dermal wounds and as a synovial lubricant. • In ophthalmology, HA is used as SOC to maintain the globe during cataract, corneal and retinal surgeries • Leading first line treatment in Europe and Asia for the ocular surface disease dry eye
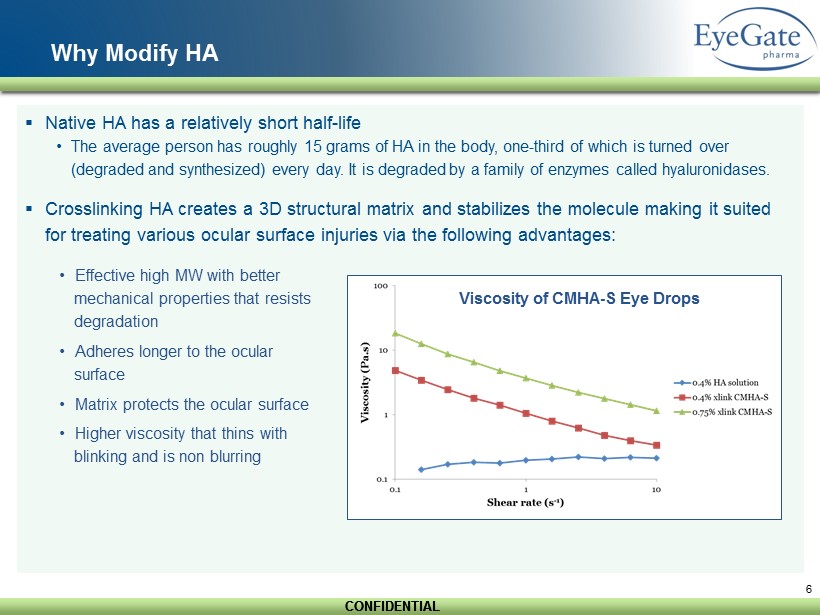
CONFIDENTIAL CONFIDENTIAL 6 Why Modify HA ▪ Native HA has a relatively short half - life • The average person has roughly 15 grams of HA in the body, one - third of which is turned over (degraded and synthesized) every day. It is degraded by a family of enzymes called hyaluronidases. ▪ Crosslinking HA creates a 3D structural matrix and stabilizes the molecule making it suited for treating various ocular surface injuries via the following advantages: • Effective high MW with better mechanical properties that resists degradation • Adheres longer to the ocular surface • Matrix protects the ocular surface • Higher viscosity that thins with blinking and is non blurring Viscosity of CMHA - S Eye Drops

CONFIDENTIAL CONFIDENTIAL 7 Real World Animal Experience ▪ CMHA - S has demonstrated global safety and efficacy in small animals in real world setting ▪ Masked studies completed demonstrating efficacy • JDE - 001 (0.4 % concentration) – Masked, randomized 20 - dog DED study: superior efficacy vs. non - cross - linked 0.4% HA in dogs that failed cyclosporine • Marketed as a highly efficacious veterinary product by Bayer Animal Health under the Remend ™ brand to treat corneal wounds (0.75% concentration) and dry eye (0.4% concentration). • http:// www.bayerdvm.com/show.aspx/remend - cross - linking - video Finnegan Bell a 5yr old cat with a non healing corneal defect • Pictured at 35 days ( left) • Healed after 10 days of using 0.75% CMHA - S TID (right) • JDE - 003 (0.75% concentration) – Efficacy demonstrated in animals in a masked study in non - healing corneal defects in dogs, and superior efficacy vs. non - cross - linked HA in a masked, randomized study of 30 cats with corneal defects
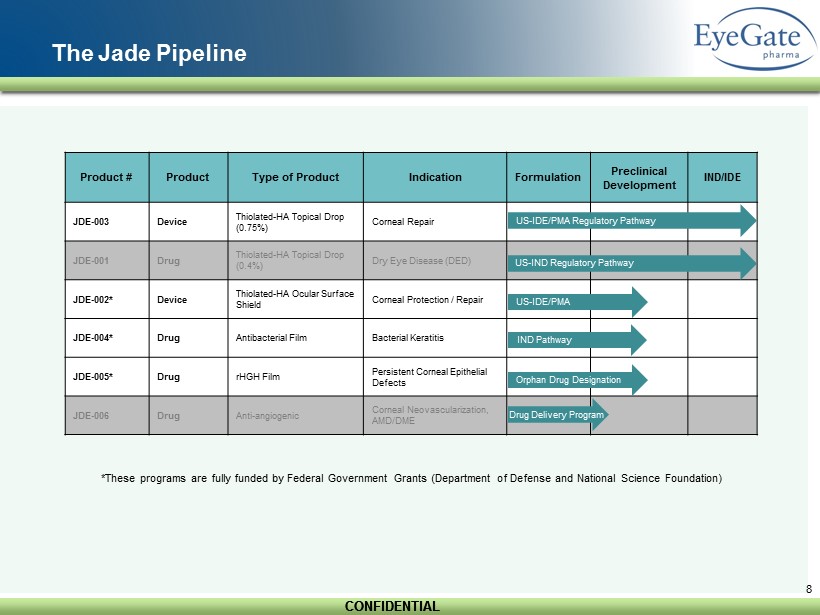
CONFIDENTIAL CONFIDENTIAL 8 The Jade Pipeline Product # Product Type of Product Indication Formulation Preclinical Development IND/IDE JDE - 003 Device Thiolated - HA Topical Drop (0.75%) Corneal Repair JDE - 001 Drug Thiolated - HA Topical Drop (0.4%) Dry Eye Disease (DED) JDE - 002* Device Thiolated - HA Ocular Surface Shield Corneal Protection / Repair JDE - 004* Drug Antibacterial Film Bacterial Keratitis JDE - 005* Drug rHGH Film Persistent Corneal Epithelial Defects JDE - 006 Drug Anti - angiogenic Corneal Neovascularization, AMD/DME US - IDE/PMA Regulatory Pathway US - IND Regulatory Pathway US - IDE/PMA IND Pathway Orphan Drug Designation Drug Delivery Program *These programs are fully funded by Federal Government Grants (Department of Defense and National Science Foundation)
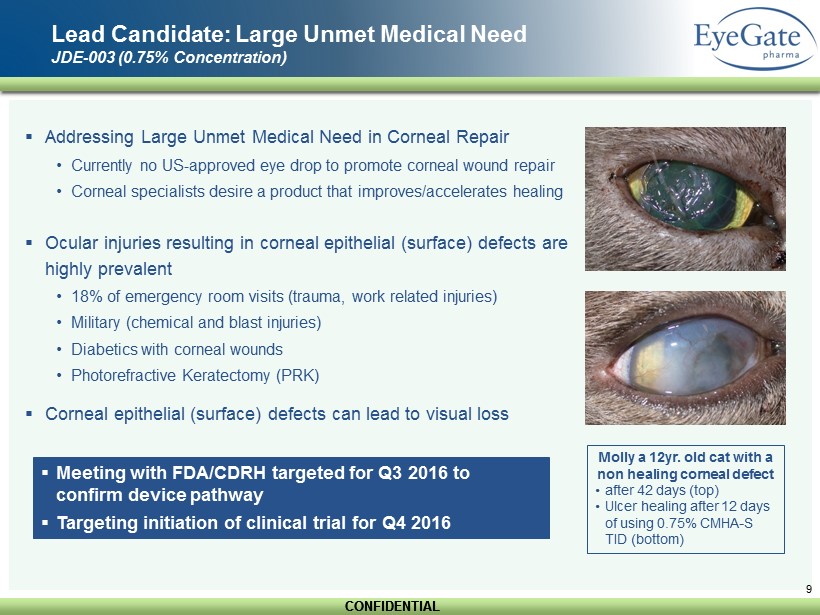
CONFIDENTIAL CONFIDENTIAL 9 Lead Candidate: Large Unmet Medical Need JDE - 003 (0.75% Concentration) ▪ Addressing Large Unmet Medical Need in Corneal Repair • Currently no US - approved eye drop to promote corneal wound repair • Corneal specialists desire a product that improves/accelerates healing ▪ Ocular injuries resulting in corneal epithelial (surface) defects are highly prevalent • 18% of emergency room visits (trauma, work related injuries) • Military (chemical and blast injuries) • Diabetics with corneal wounds • Photorefractive Keratectomy (PRK ) ▪ Corneal epithelial (surface) defects can lead to visual loss Molly a 12yr. old cat with a non healing corneal defect • after 42 days (top) • Ulcer healing after 12 days of using 0.75% CMHA - S TID (bottom) ▪ Meeting with FDA/CDRH targeted for Q3 2016 to confirm device pathway ▪ Targeting initiation of clinical trial for Q4 2016

CONFIDENTIAL CONFIDENTIAL 10 Lead Candidate: Competition JDE - 003 (0.75% Concentration) ▪ AmbioDisk TM from IOP Ophthalmics ) is an amniotic membrane (AM ) device - a sutureless , overlay AM disk for the office - based or surgical treatment of the ocular surface. ($800/ treatment) ▪ Prokera ® (from BioTissue ) is a cryopreserved AM on a ring, occasionally sutured in the OR or office for non healing corneal disease ($1800/ treatment) ▪ AmbioDisk & Prokera are used for Corneal Defects: • Non healing corneal wounds/ ulcers • Neurotrophic Ulcerations • Corneal Erosions • Acute Chemical/Thermal Burns • Post - Infectious Keratitis (herpetic, vernal, bacterial)
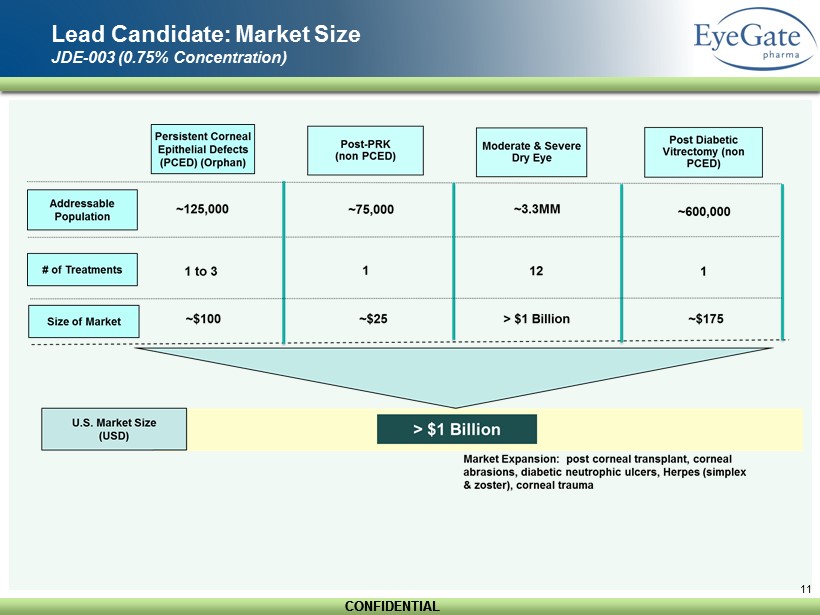
CONFIDENTIAL CONFIDENTIAL 11 Lead Candidate: Market Size JDE - 003 (0.75% Concentration)

CONFIDENTIAL CONFIDENTIAL 12 Ocular Surface Shield ▪ Medical Need: A sterile, field - stable product that is easily applied to immediately protect and promote healing of the ocular surface (Collagen Bandage Contact Lens) ▪ Design : A thin film, contoured to the shape of the globe/ocular surface, that will remain in place during the healing process; prevent adhesions and be able to deliver medications topically ▪ Desired Properties of the Film: • Easy to place, requiring no sutures or glue • Allows for immediate stabilization of the eye following trauma • Remains in place during the initial healing process, and up to 7 days • Promotes ocular tissue repair • Prevents adhesions and scar formation between the globe and the conjunctiva • Improved version of biologic “Amniotic Membranes” ▪ Funding : DoD SBIR – Phase 1 & 2 (funding in place through 8/2017)
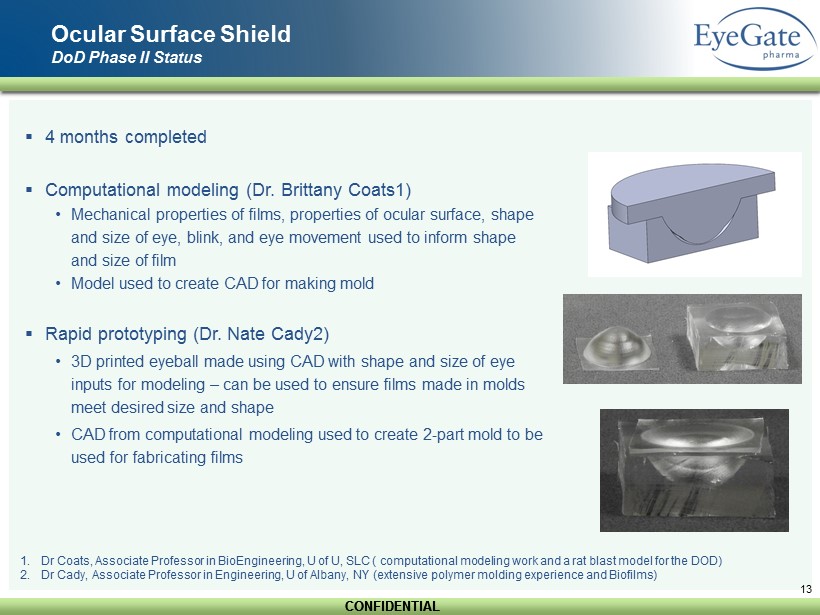
CONFIDENTIAL CONFIDENTIAL 13 Ocular Surface Shield DoD Phase II Status ▪ 4 months completed ▪ Computational modeling (Dr. Brittany Coats1) • Mechanical properties of films, properties of ocular surface, shape and size of eye, blink, and eye movement used to inform shape and size of film • Model used to create CAD for making mold ▪ Rapid prototyping (Dr. Nate Cady2) • 3D printed eyeball made using CAD with shape and size of eye inputs for modeling – can be used to ensure films made in molds meet desired size and shape • CAD from computational modeling used to create 2 - part mold to be used for fabricating films 1. Dr Coats, Associate Professor in BioEngineering , U of U, SLC ( computational modeling work and a rat blast model for the DOD) 2. Dr Cady, Associate Professor in Engineering, U of Albany, NY (extensive polymer molding experience and Biofilms)

CONFIDENTIAL CONFIDENTIAL 14 Topical Sustained - Release Delivery Antibiotics: Bacterial Keratitis/Conjunctivitis ▪ A corneal blinding condition (Scarring/perforation) ▪ Most common cause in US is contact lens wear ▪ Standard of care is topical antibiotics dosed as often as hourly ▪ No marketed drug product in the US ▪ NSF SBIR grant + Army interest ▪ 7 - 8 day release from CHMA film Focus ▪ Current Focus: Besifloxacin - HCl (solubility, 1 mg/ml) • Besivance ® ( Bausch+Lomb ) approved for bacterial conjunctivitis
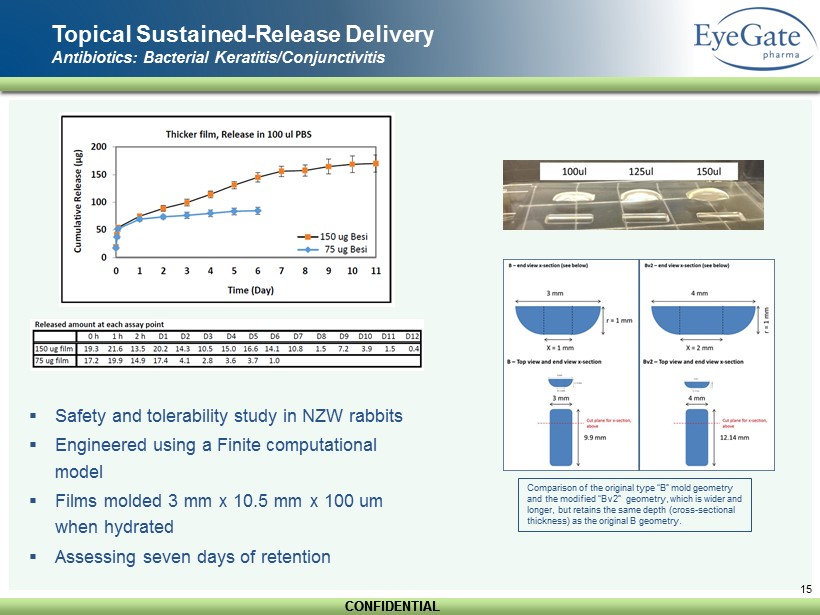
CONFIDENTIAL CONFIDENTIAL 15 Topical Sustained - Release Delivery Antibiotics: Bacterial Keratitis/Conjunctivitis ▪ Safety and tolerability study in NZW rabbits ▪ Engineered using a Finite computational model ▪ Films molded 3 mm x 10.5 mm x 100 um when hydrated ▪ Assessing seven days of retention Comparison of the original type “B” mold geometry and the modified “Bv2 ” geometry, which is wider and longer, but retains the same depth ( cross - sectional thickness) as the original B geometry .