EXHIBIT 99.1
Published on January 9, 2023
Exhibit 99.1

Kiora Pharmaceuticals, Inc. NASDAQ: KPRX January 2023 | Corporate Outlook

2 Forward Looking Statements Some of the statements in this presentation are “forward - looking” and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995 . These “forward - looking” statements include statements relating to, among other things, the development and commercialization efforts and other regulatory or marketing approval efforts pertaining to Kiora’s products, including KIO - 101 , KIO - 201 and KIO - 301 , as well as the success thereof, with such approvals or success may not be obtained or achieved on a timely basis or at all . These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this presentation, including, among other things, market and other conditions and certain risk factors described under the heading “Risk Factors” contained in Kiora’s Amended Annual Report on Form 10 - K/A filed with the SEC on July 7 , 2022 , or described in Kiora’s other public filings . Kiora’s results may also be affected by factors of which Kiora is not currently aware . The forward - looking statements in this presentation speak only as of the date of this presentation . Kiora expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions, or circumstances on which any such statement is based, except as required by law .

Developing Therapeutics for Rare & Underserved Ophthalmic Diseases Priority Asset – KIO - 301: Vision Restoration in Retinitis Pigmentosa (RP) ▪ Small molecule “photoswitch” is gene mutation agnostic, easy to deliver ▪ Dosed 1 st patient Q4 2022 in Phase 1b study ▪ Anticipate preliminary results 1H 2023 KIO - 101: Ocular Surface Disease in Rheumatoid Arthritis & Other Autoimmune Diseases (OPRA+) ▪ Investigational New Drug Application for Phase 2 trial conditionally approved ▪ Disease modifying approach ▪ First patient, first visit planned for 1H 2023 KIO - 201: A Novel, Modified Hyaluronic Acid (HA) Molecule for Ocular Wound Healing ▪ Phase 2 Persistent Corneal Epithelial Defects (PCED) trial (orphan indication) fully enrolled in Q4 2022 ▪ Report results & initiate discussions with FDA for registration trial 1H 2023 3 Corporate Highlights
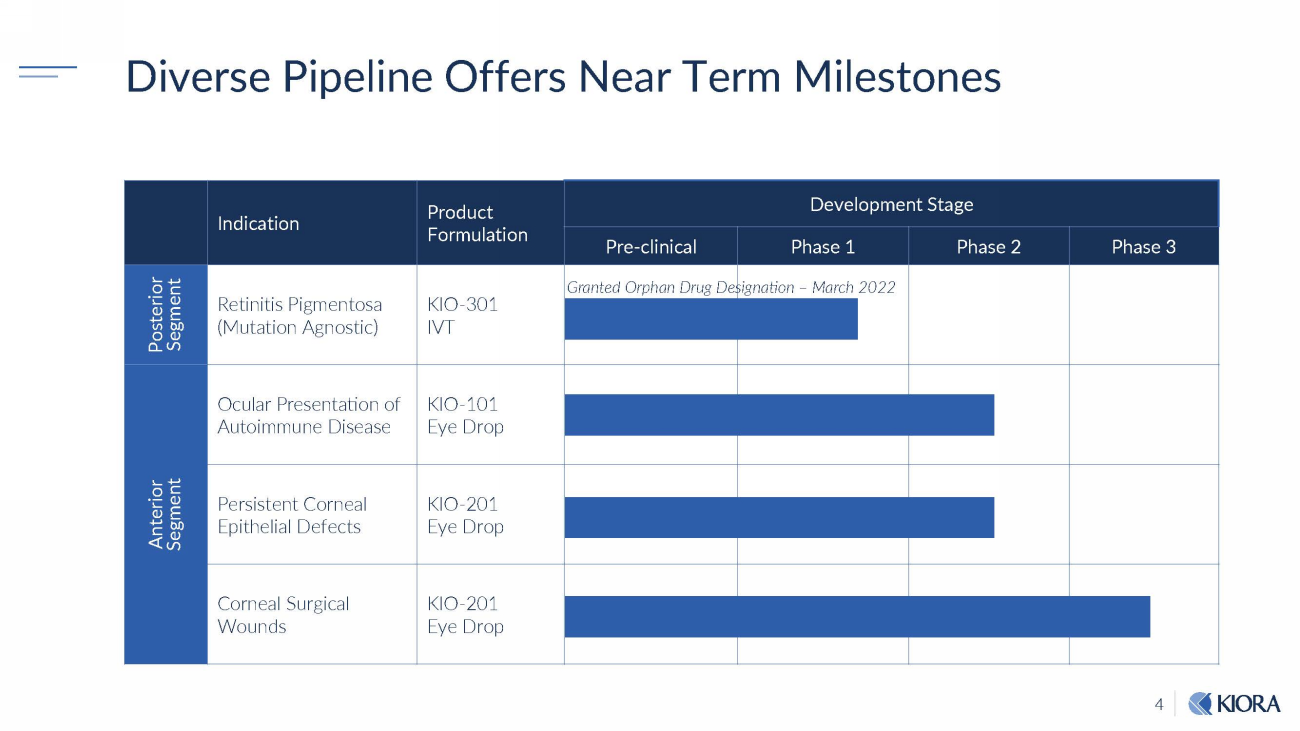
4 S egme n t Diverse Pipeline Offers Near Term Milestones Indication Product F o rm u lati o n Development Stage Pre - clinical Phase 1 Phase 2 Phase 3 P o st e ri o r Segment Retinitis Pigmentosa (Mutation Agnostic) K I O - 301 IVT Granted Orphan Drug De signation – March 2022 Anterior Ocular Presentation of Autoimmune Disease KIO - 101 Eye Drop Persistent Corneal Epithelial Defects KIO - 201 Eye Drop Corneal Surgical Wounds KIO - 201 Eye Drop

Achieved Several Clinical and Regulatory Development Milestones ▪ Initiated ABACUS, a first - in - human Phase 1b trial for KIO - 301 in RP ▪ Received Orphan Drug Designation (ODD) for KIO - 301 in RP ▪ Initiated and completed enrollment of Phase 2 trial of KIO - 201 for PCED ▪ Reported full results from KIO - 101 Phase 1b trial in ocular surface inflammation ▪ Investigational New Drug Application conditionally approved for Phase 2 trial for KIO - 101 in OPRA+ Implemented Operational and Financial Efficiencies & Strengthened Financial and Reporting Procedures ▪ Appointed Melissa Tosca as EVP Finance to oversee finance, reporting, and accounting ▪ Identified and updated gaps in legacy SEC reporting protocols ▪ Received ~$1.2M in refundable R&D tax credits ▪ Raised gross $20 million (past 18 months) 5 Milestones 2022 Recap: Strategic Focus Pi p e l ine Sharpened Pipeline ▪ Targeting rare and/or differentiated ophthalmic indications ▪ Pursuing cost - effective paths to market Fi n ance

6 KIO - 301 Vision Restoration in Retinitis Pigmentosa

7 Prevalence ▪ 1 : 3,500 worldwide ▪ Approximate 100,000 in US Etiology ▪ 50+ genetically distinct subtypes from 150+ mutations ▪ Inherited disease Clinical Presentation ▪ Night blindness, reduced visual field range and eventual loss of central vision ▪ Visual acuity declines Diagnosis ▪ Retinal exam (black bone - spicule pigmentation) ▪ ERG provides definitive diagnosis ▪ Genetic testing Targeting RP A Condition with No Available Treatments American Academy of Ophthalmology Normal Vision Vision Declines over Time
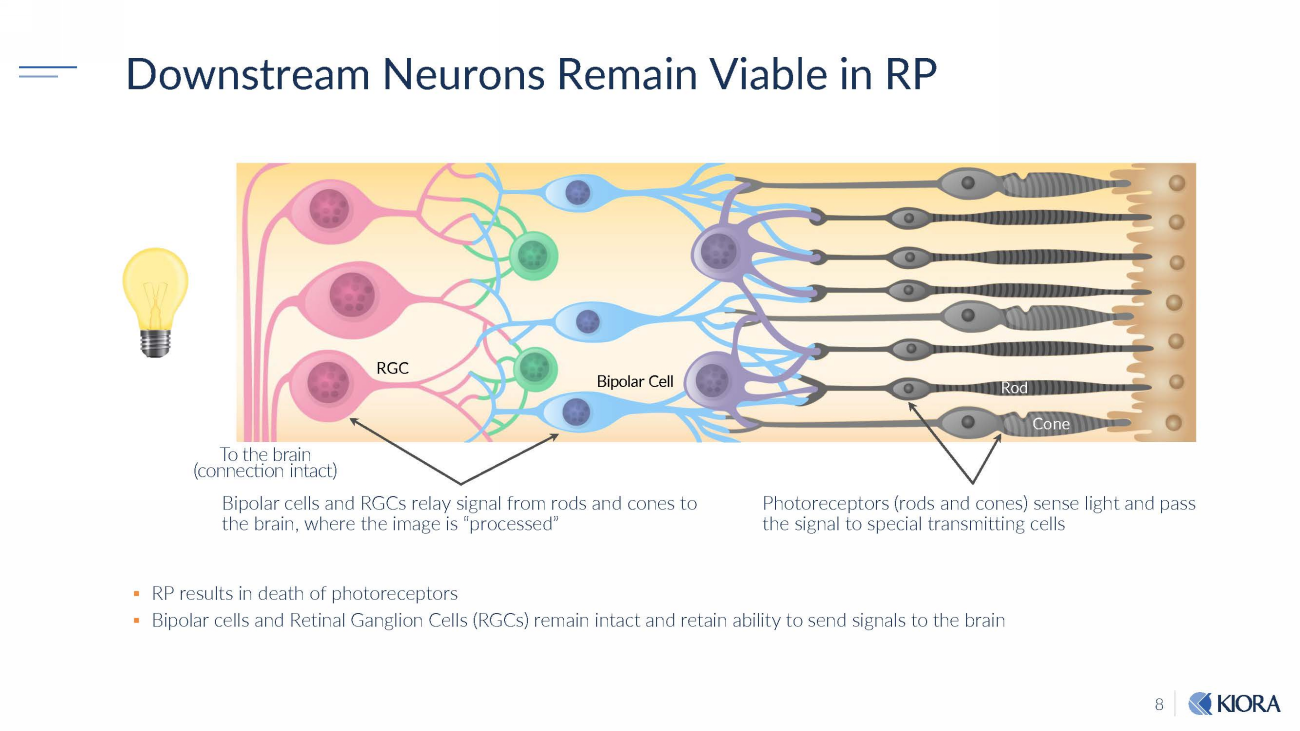
8 Downstream Neurons Remain Viable in RP ▪ RP results in death of photoreceptors ▪ Bipolar cells and Retinal Ganglion Cells (RGCs) remain intact and retain ability to send signals to the brain Photoreceptors (rods and cones) sense light and pass the signal to special transmitting cells R GC Bipolar Cell To the brain (connection intact) Bipolar cells and RGCs relay signal from rods and cones to the brain, where the image is “processed” R od Co ne
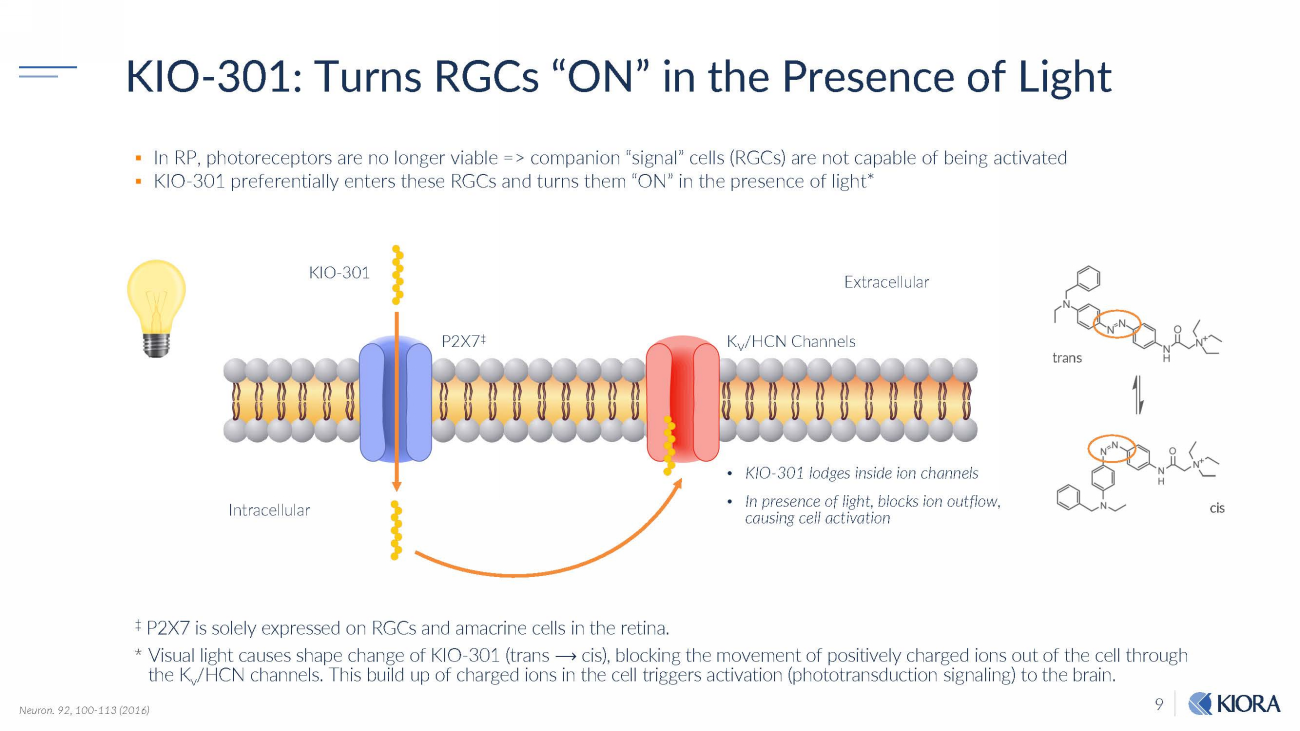
9 Neuron. 92, 100 - 113 (2016) tr an s ci s ▪ In RP, photoreceptors are no longer viable => companion “signal” cells (RGCs) are not capable of being activated ▪ KIO - 301 preferentially enters these RGCs and turns them “ON” in the presence of light* ‡ P2X7 is solely expressed on RGCs and amacrine cells in the retina. * Visual light causes shape change of KIO - 301 (trans ⟶ cis), blocking the movement of positively charged ions out of the cell through the K v /HCN channels. This build up of charged ions in the cell triggers activation (phototransduction signaling) to the brain. In t r a c ell ul a r K I O - 301 P2X7 ‡ Ext r a c e llul a r K V /HCN Channels • KIO - 301 lodges inside ion channels KIO - 301: Turns RGCs “ON” in the Presence of Light • In presence of light, blocks ion outflow, causing cell activation
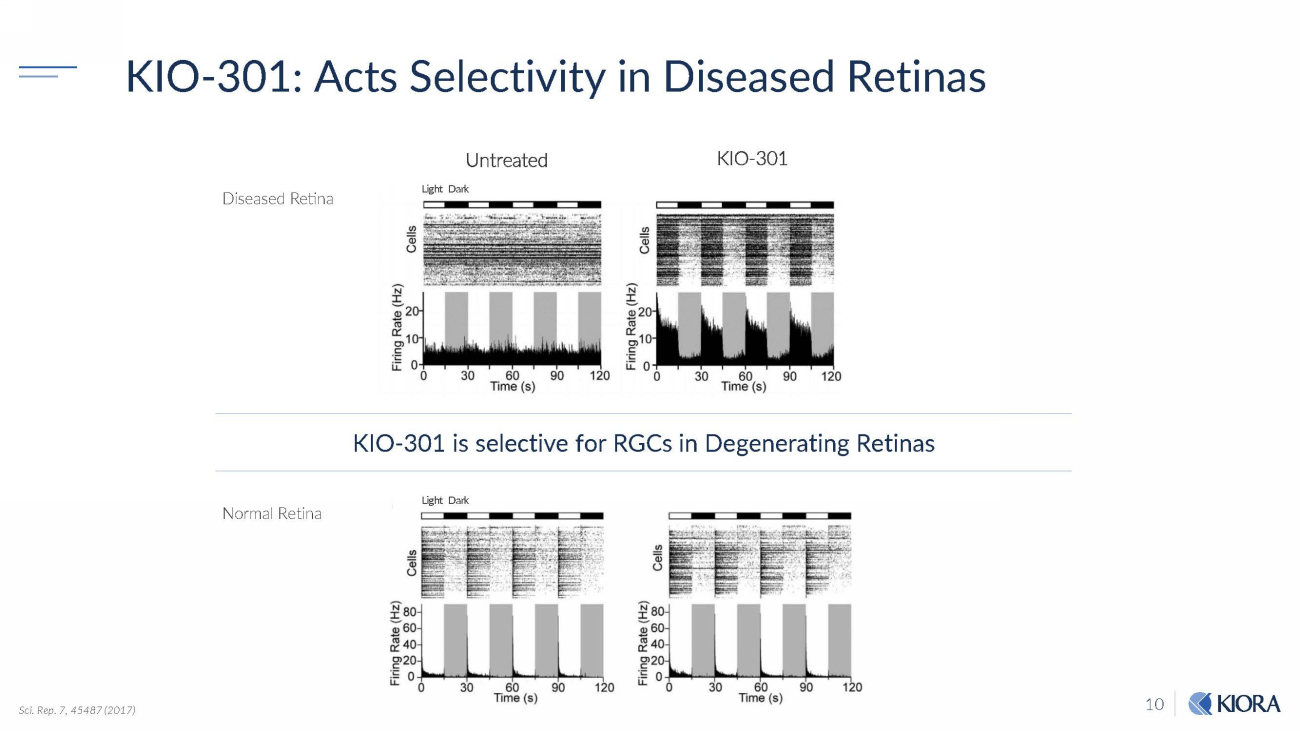
10 KIO - 301: Acts Selectivity in Diseased Retinas Sci. Rep. 7, 45487 (2017) KIO - 301 is selective for RGCs in Degenerating Retinas Normal Retina Diseased Retina Light Dark Light Dark U n t r e a t e d KIO - 301
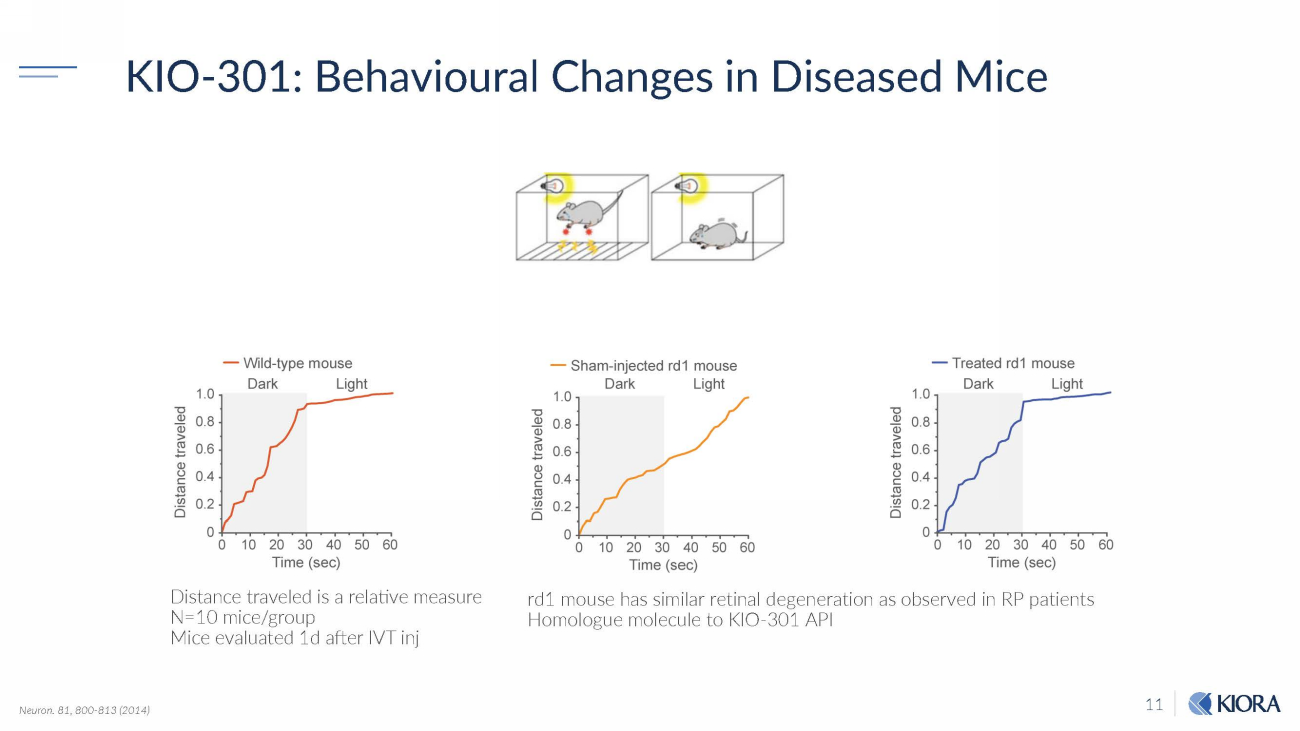
11 KIO - 301: Behavioural Changes in Diseased Mice rd1 mouse has similar retinal degeneration as observed in RP patients Homologue molecule to KIO - 301 API Distance traveled is a relative measure N=10 mice/group Mice evaluated 1d after IVT inj Neuron. 81, 800 - 813 (2014)
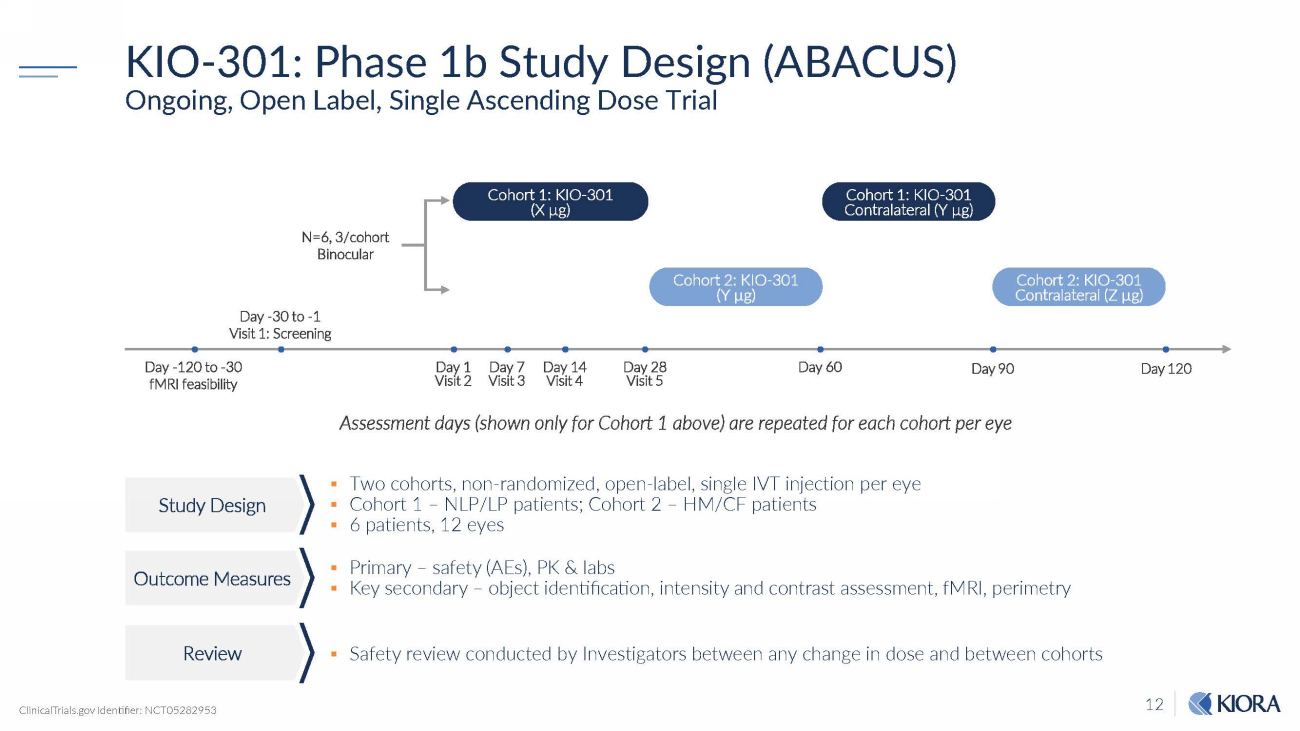
12 KIO - 301: Phase 1b Study Design (ABACUS) Ongoing, Open Label, Single Ascending Dose Trial ▪ Two cohorts, non - randomized, open - label, single IVT injection per eye ▪ Cohort 1 – NLP/LP patients; Cohort 2 – HM/CF patients ▪ 6 patients, 12 eyes ▪ Primary – safety (AEs), PK & labs ▪ Key secondary – object identification, intensity and contrast assessment, fMRI, perimetry ▪ Safety review conducted by Investigators between any change in dose and between cohorts N=6, 3/cohort Binocular Day 90 Cohort 1: KIO - 301 (X µg) Day - 30 to - 1 Visit 1: Screening Day 28 Visit 5 Day 60 Day 120 Cohort 1: KIO - 301 Contralateral (Y µg) Cohort 2: KIO - 301 (Y µg) Cohort 2: KIO - 301 Contralateral (Z µg) Day 1 Visit 2 Day 7 Visit 3 Day 14 Visit 4 Day - 120 to - 30 fMRI feasibility ClinicalTrials.gov Identifier: NCT05282953 Assessment days (shown only for Cohort 1 above) are repeated for each cohort per eye Study Design Outcome Measures R e v i e w
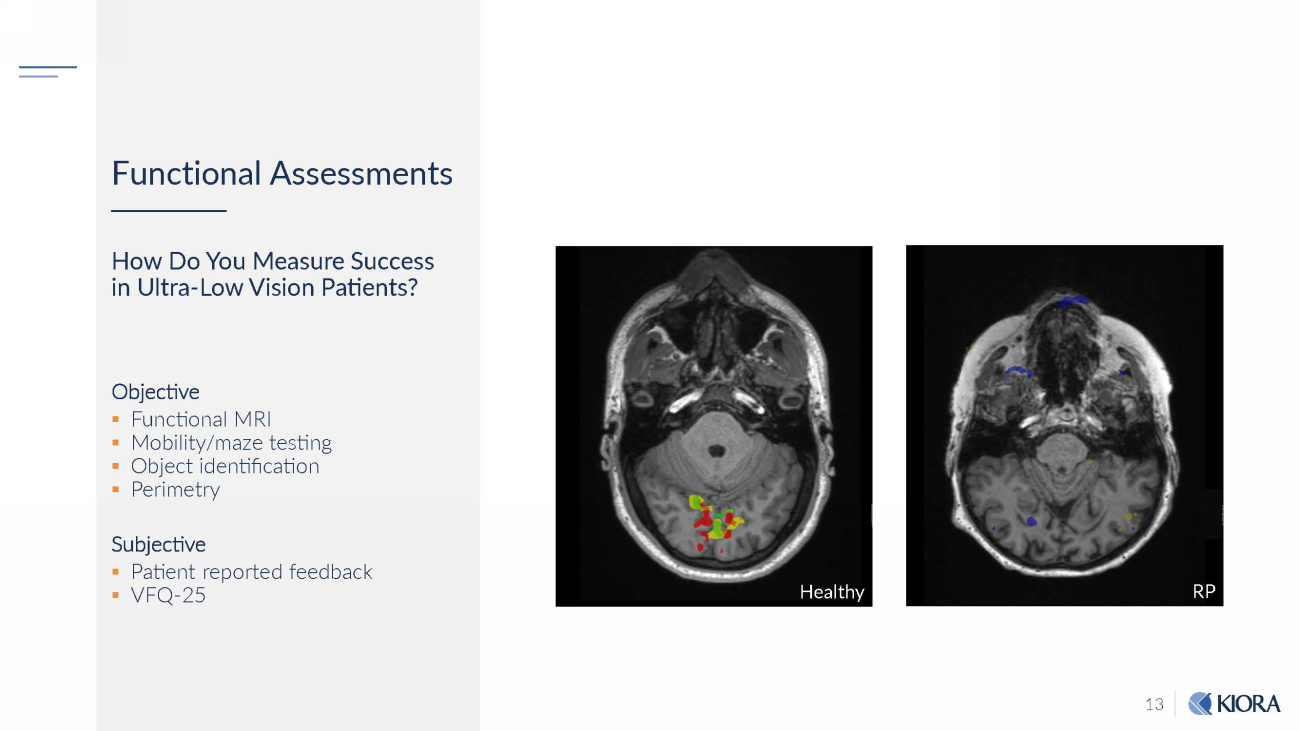
13 Functional Assessments How Do You Measure Success in Ultra - Low Vision Patients? Objective ▪ Functional MRI ▪ Mobility/maze testing ▪ Object identification ▪ Perimetry Subjective ▪ Patient reported feedback ▪ VFQ - 25 H eal thy RP
14 Beyond RP for Photoswitch Platform ▪ Other inherited retinal diseases (rod - cone dystrophies) ▪ Age - related macular degenerative diseases > Geographic atrophy > Late - stage wAMD ▪ In combination with any and all gene - replacement therapies ▪ Screening for optogenetics Indications Expanding Exclusivity ▪ Protected through at least 2041 with combination of formulation, methods, and CoM patents ▪ Orphan Drug Designation regulatory protection

15 KIO - 101 Ocular Presentation of Rheumatoid Arthritis & Other Autoimmune Diseases (OPRA+)
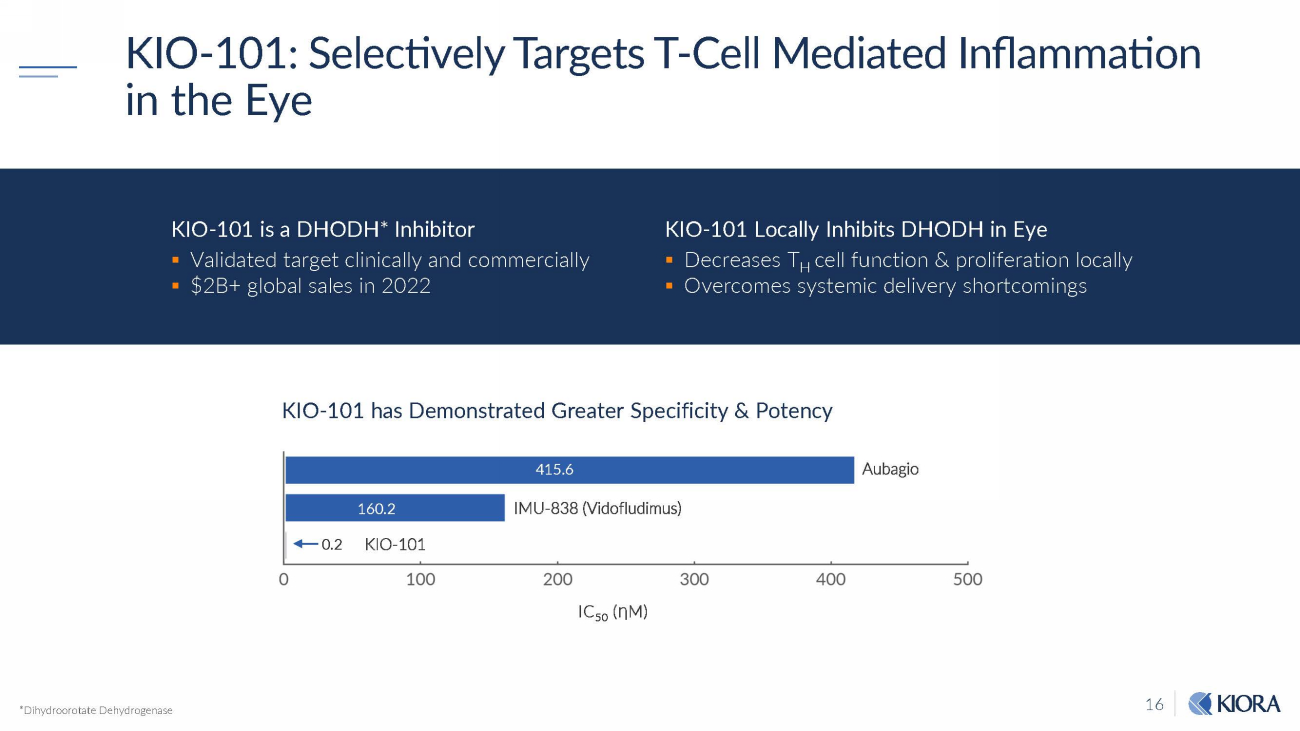
16 KIO - 101: Selectively Targets T - Cell Mediated Inflammation in the Eye *Dihydroorotate Dehydrogenase KIO - 101 has Demonstrated Greater Specificity & Potency IMU - 838 (Vidofludimus) A u b a g i o 415 . 6 160 . 2 0.2 KIO - 101 IC 50 ( Ƞ M) KIO - 101 Locally Inhibits DHODH in Eye ▪ Decreases T H cell function & proliferation locally ▪ Overcomes systemic delivery shortcomings KIO - 101 is a DHODH* Inhibitor ▪ Validated target clinically and commercially ▪ $2B+ global sales in 2022
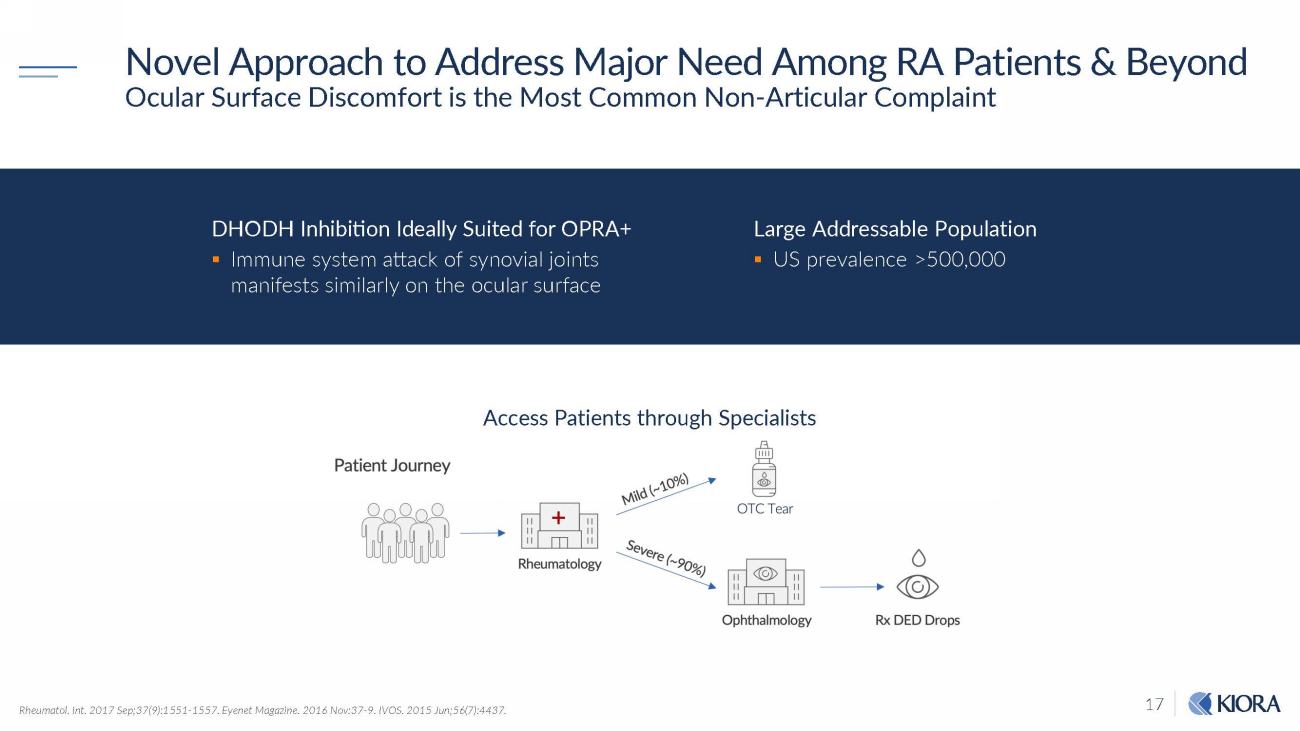
17 Novel Approach to Address Major Need Among RA Patients & Beyond Ocular Surface Discomfort is the Most Common Non - Articular Complaint Rheumatol. Int. 2017 Sep;37(9):1551 - 1557. Eyenet Magazine. 2016 Nov:37 - 9. IVOS. 2015 Jun;56(7):4437. Access Patients through Specialists DHODH Inhibition Ideally Suited for OPRA+ ▪ Immune system attack of synovial joints manifests similarly on the ocular surface Large Addressable Population ▪ US prevalence >500,000
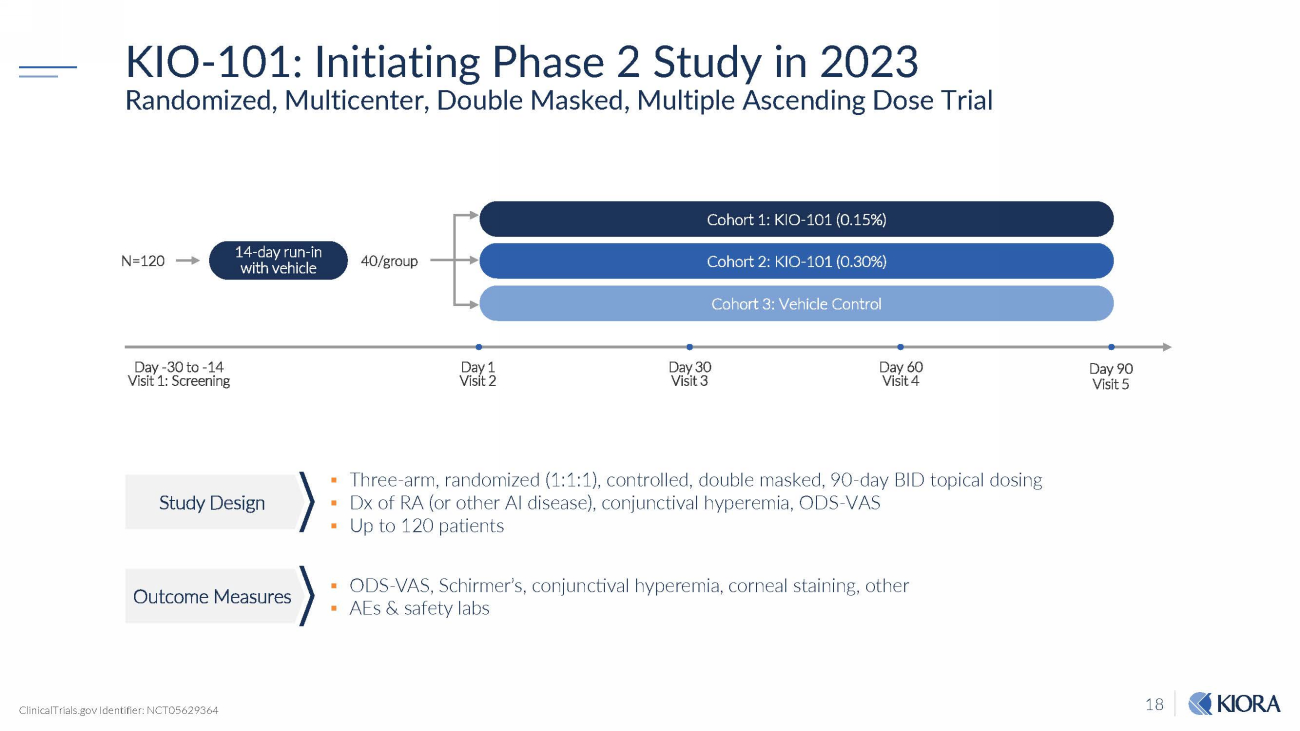
18 KIO - 101: Initiating Phase 2 Study in 2023 Randomized, Multicenter, Double Masked, Multiple Ascending Dose Trial ▪ Three - arm, randomized (1:1:1), controlled, double masked, 90 - day BID topical dosing ▪ Dx of RA (or other AI disease), conjunctival hyperemia, ODS - VAS ▪ Up to 120 patients ▪ ODS - VAS, Schirmer’s, conjunctival hyperemia, corneal staining, other ▪ AEs & safety labs N = 120 Day 90 Visit 5 Cohort 1: KIO - 101 (0.15%) Cohort 2: KIO - 101 (0.30%) Day 1 Visit 2 Cohort 3: Vehicle Control Day - 30 to - 14 Visit 1: Screening Day 30 Visit 3 Day 60 Visit 4 14 - day run - in with vehicle 40/group ClinicalTrials.gov Identifier: NCT05629364 Study Design Outcome Measures

19 KIO - 201 Proprietary, Modified Form of Hyaluronic Acid (HA) to Heal Challenging Ocular Surface Wounds

20 Ocular Surface Wounds: Opportunity Set Persistent Corneal Epithelial Defects (PCED) Ocular Surface Surgical Recovery ▪ Failure of normal closure of a corneal injury in 10 – 14 days, despite standard of care ▪ Orphan Drug Designation (ODD) opportunity ▪ PRK – Surgical correction of refractive errors for patients who are not candidates for LASIK > Standard of care is the use of bandage contact lens that can result in erosion of epithelium ▪ Keratoconus – Rare ocular disease where the cornea thins and bulges outward > Treatment option includes corneal cross - linking, a laser surgical procedure Med. Hypothesis Discov. Innov. Ophthalmol. 8 (2019): 163 - 176.
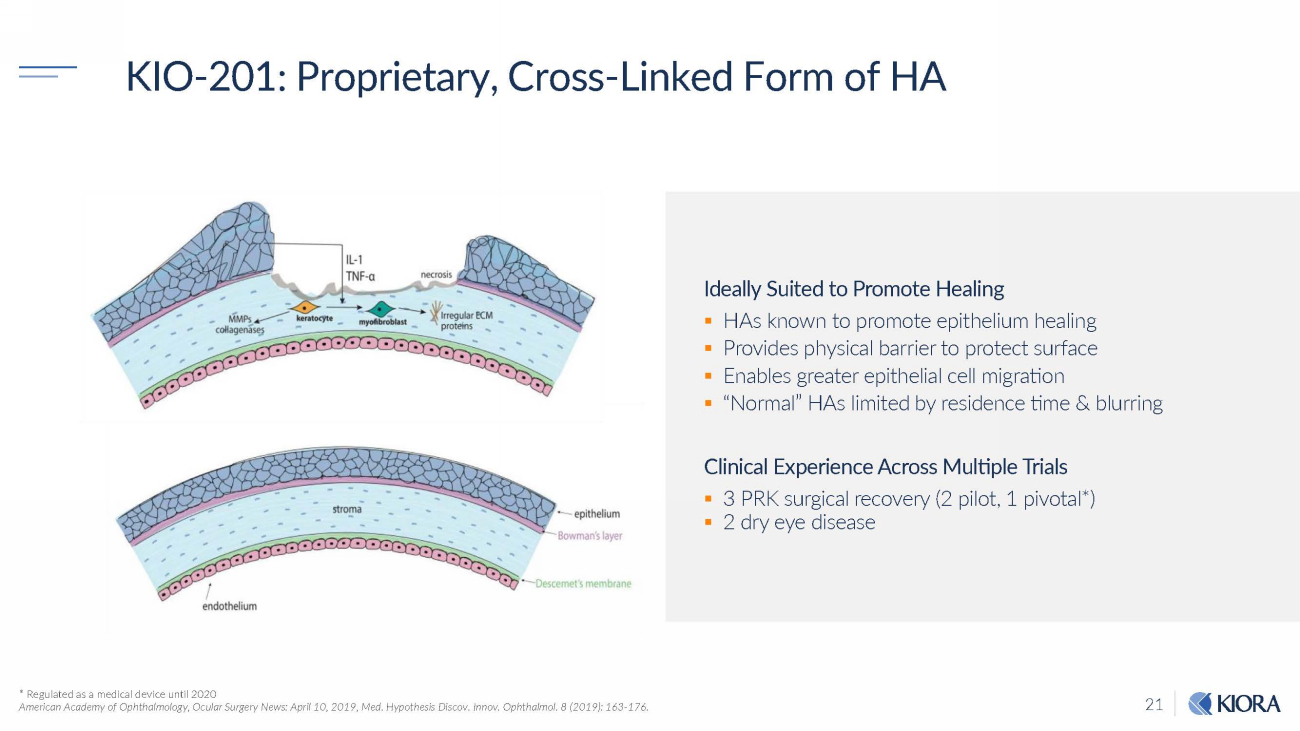
21 KIO - 201: Proprietary, Cross - Linked Form of HA * Regulated as a medical device until 2020 American Academy of Ophthalmology, Ocular Surgery News: April 10, 2019, Med. Hypothesis Discov. Innov. Ophthalmol. 8 (2019): 163 - 176. Ideally Suited to Promote Healing ▪ HAs known to promote epithelium healing ▪ Provides physical barrier to protect surface ▪ Enables greater epithelial cell migration ▪ “Normal” HAs limited by residence time & blurring Clinical Experience Across Multiple Trials ▪ 3 PRK surgical recovery (2 pilot, 1 pivotal*) ▪ 2 dry eye disease
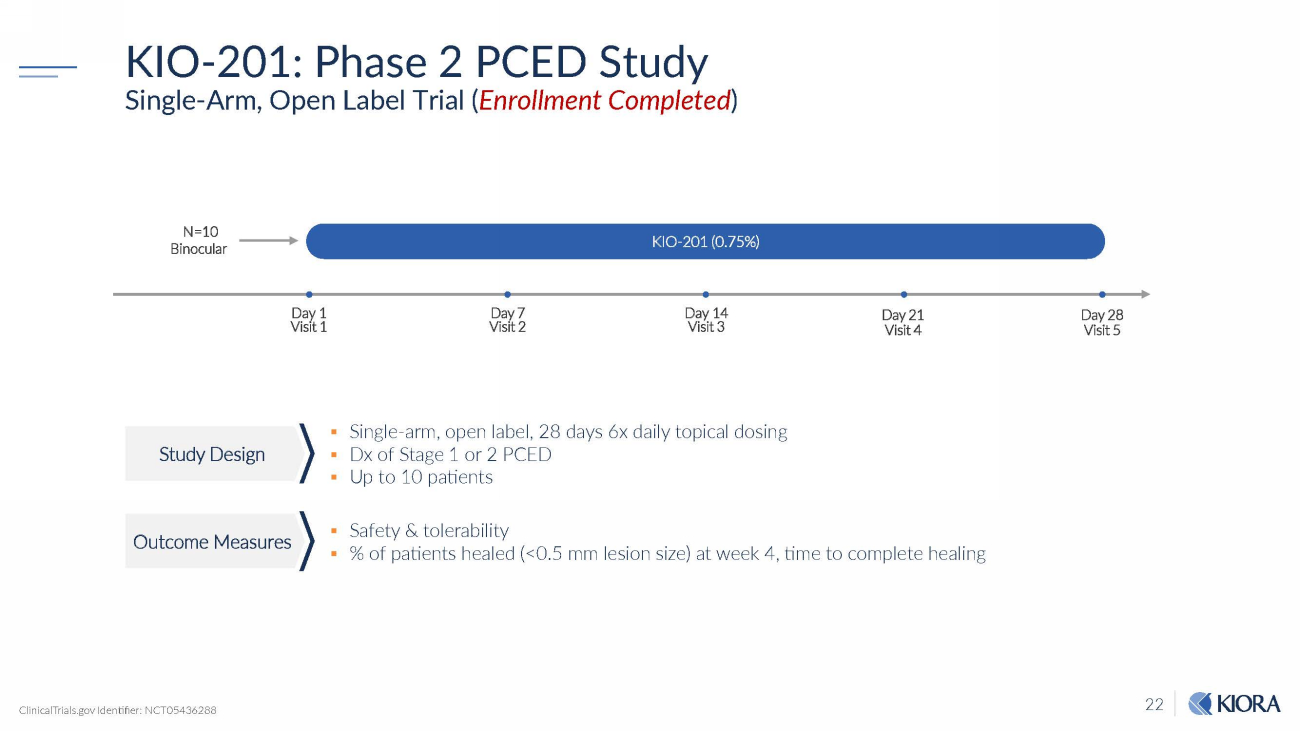
22 KIO - 201: Phase 2 PCED Study Single - Arm, Open Label Trial ( Enrollment Completed ) ▪ Single - arm, open label, 28 days 6x daily topical dosing ▪ Dx of Stage 1 or 2 PCED ▪ Up to 10 patients ▪ Safety & tolerability ▪ % of patients healed (<0.5 mm lesion size) at week 4, time to complete healing Day 21 Visit 4 KIO - 201 (0.75%) Day 1 Visit 1 Day 7 Visit 2 Day 14 Visit 3 N=10 B in o c u l a r Day 28 Visit 5 ClinicalTrials.gov Identifier: NCT05436288 Study Design Outcome Measures

23 CORPORATE OVERVIEW Management & Milestones
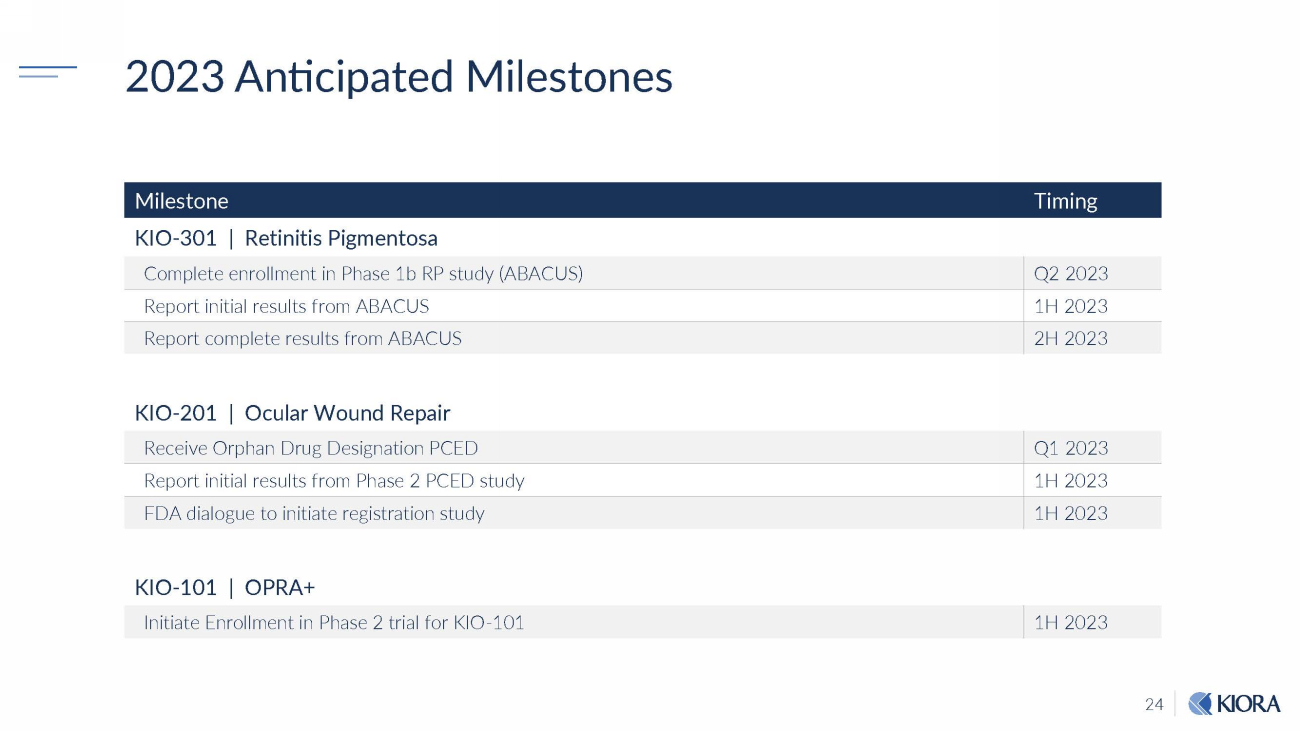
24 2023 Anticipated Milestones Milestone Timing KIO - 301 | Retinitis Pigmentosa Complete enrollment in Phase 1b RP study (ABACUS) Q2 2023 Report initial results from ABACUS 1H 2023 Report complete results from ABACUS 2H 2023 KIO - 201 | Ocular Wound Repair Receive Orphan Drug Designation PCED Q1 2023 Report initial results from Phase 2 PCED study 1H 2023 FDA dialogue to initiate registration study 1H 2023 KIO - 101 | OPRA+ Initiate Enrollment in Phase 2 trial for KIO - 101 1H 2023

25 Leadership Team Brian M Strem, PhD President & CEO Stefan Sperl, PhD EVP – CMC & Operations Angela Dentiste, MBA VP – Clinical Operations Eric J Daniels, MD, MBA Chief Development Officer Melissa Tosca, CPA EVP – Finance

26 Board of Directors Brian M Strem, PhD President & CEO Paul Chaney Chairman Aron Shapiro Ken Gayron Praveen Tyle David Hollander, MD, MBA Erin Parsons

27 Scientific Advisory Board Daniel Durrie, MD Russel Van Gelder, MD, PhD Charlie Wykoff, MD Victor Perez, MD Francis Mah, MD Paul Karpecki, OD, FAAO Allen Ho, MD, PhD Christine Kay, MD, PhD Vitreo Retinal ASSOCIATES

Contact: info@kiorapharma.com