EXHIBIT 99.1
Published on September 14, 2021
Exhibit 99.1

EyeGate Pharmaceuticals (NASDAQ: EYEG) Sept 2021

2 Forward Looking Statements Some of the statements are “forward - looking” and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995 . These “forward - looking” statements include statements relating to, among other things, the commercialization efforts and other regulatory or marketing approval efforts pertaining to EyeGate’s products, including EyeGate’s PP - 001 and OBG products, as well as the success thereof, with such approvals or success may not be obtained or achieved on a timely basis or at all, the ability of EyeGate to complete the acquisition of Bayon Therapeutics in a timely manner or at all, and the results and potential benefits of the acquisition of Bayon Therapeutics, which will be subject to the receipt of all necessary approvals and satisfaction of all closing conditions for the completion of the transaction . These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this presentation, including, among other things, certain risk factors described under the heading “Risk Factors” contained in EyeGate’s Annual Report on Form 10 - K filed with the SEC on March 25 , 2021 or described in EyeGate’s other public filings . EyeGate’s results may also be affected by factors of which EyeGate is not currently aware . The forward - looking statements in this presentation speak only as of the date of this presentation . EyeGate expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions or circumstances on which any such statement is based .

3 • Pipeline in ophthalmology transformed by Panoptes acquisition and Bayon pending acquisition • PP - 001 is a best - in - class DHODH inhibitor • B - 203 is a small molecule capable of conferring light sensitivity to degenerating retinas* Corporate Overview • PP - 001 Phase 2 PoC trial in Dry Eye Disease ongoing • OBG targeting Phase 3b readiness for accelerating PRK recovery • B - 203 first - in - man trial expected to initiate in Q2 2022* Clinical Stage • PP - 001 for non - core indications ( ie autoimmune diseases) • OBG commercialization Partnership Opportunities Mergers Create Expanded Pipeline EYEG NASDAQ listed • EyeGate is an ophthalmic specialty pharmaceutical company Focus * - Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation

4 Corporate Overview PP - 001: 4 th gen small - molecule Dihydroorotate Dehydrogenase (DHODH) inhibitor – Validated immunomodulatory class, approved for RA (Sanofi - Arava) and MS (Sanofi - Aubagio) – Best - in - class with picomolar potency with no off - target side effects of prior generations EyeGate Pharmaceuticals is a clinical - stage company with unique platforms OBG: modified form of Hyaluronic Acid (HA) – Eye drop that promotes wound healing and provides lubrication – Ph3 clinical study demonstrated accelerated wound healing in PRK patients B - 203: novel small molecule photoswitch * – Confers light sensitivity to sections of the retina with upstream degenerated photoreceptors – Entering Ph1b trials in late - stage RP expected in Q2 2022 * - Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation
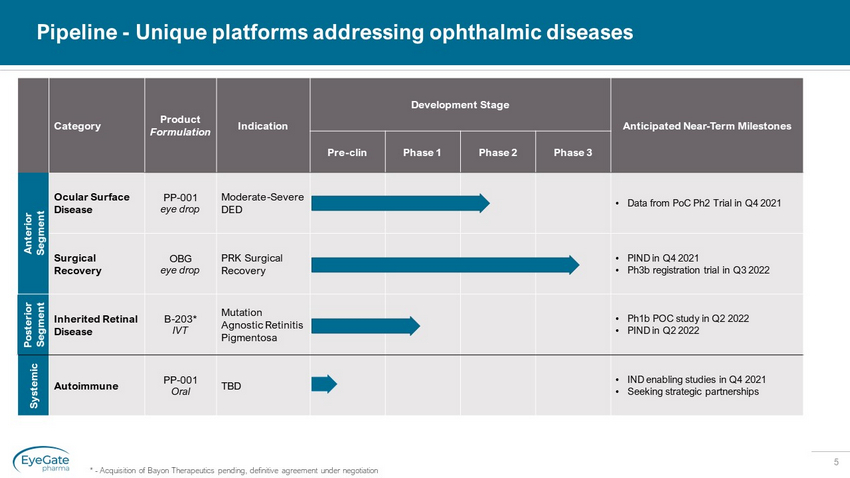
5 Pipeline - Unique platforms addressing ophthalmic diseases Category Product Formulation Indication Development Stage Anticipated Near - Term Milestones Pre - clin Phase 1 Phase 2 Phase 3 Anterior Segment Ocular Surface Disease PP - 001 eye drop Moderate - Severe DED • Data from PoC Ph2 Trial in Q4 2021 Surgical Recovery OBG eye drop PRK Surgical Recovery • PIND in Q4 2021 • Ph3b registration trial in Q3 2022 Posterior Segment Inherited Retinal Disease B - 203* IVT Mutation Agnostic Retinitis Pigmentosa • Ph1b POC study in Q2 2022 • PIND in Q2 2022 Systemic Autoimmune PP - 001 Oral TBD • IND enabling studies in Q4 2021 • Seeking strategic partnerships * - Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation

PP - 001 DHODH Inhibitor
7 Dry Eye: Opportunity • A multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film – Inflammation is the common denominator of pathogenesis • Significantly affects the quality of life – Chronicity, pain and irritation, blindness in severe form • Tens of millions worldwide – 9 million 1 people in U.S. have the moderate/severe form of DED • No definitive treatment that works in most patients: only ~1.6 out of 9 million patients are being treated – Cyclosporine 0.05% (Restasis®, Allergan - 2020 US sales $1.3 billion 2 ) – Lifitegrast 5% (Xiidra®, Shire - 2020 US sales of $376M 3 ) – Steroids • To date only anti - inflammatories have been approved by FDA – Will require multiple mechanisms to satisfy patient population – Xiidra’s introduction in 2016 helped increase overall prescribing 4 • Rx’s went from ~3mn to ~4mn per annum (1.4mn unique patients in 2020) 1. Global Data's Dry Eye Syndrome Global Drug Forecast and Market Analysis to 2026: Published June 2018 2. Based on Q4 2020 run rate reported by Abbvie 3. 2020 sales reported by Novartis 4. Symphony Health – OIS presentation 2020
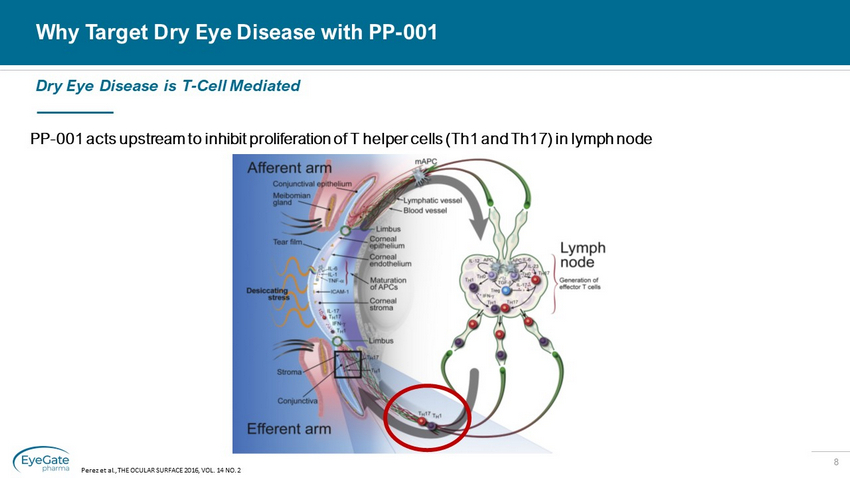
8 Why Target Dry Eye Disease with PP - 001 PP - 001 acts upstream to inhibit proliferation of T helper cells (Th1 and Th17) in lymph node Dry Eye Disease is T - Cell Mediated Perez et al., THE OCULAR SURFACE 2016, VOL. 14 NO. 2
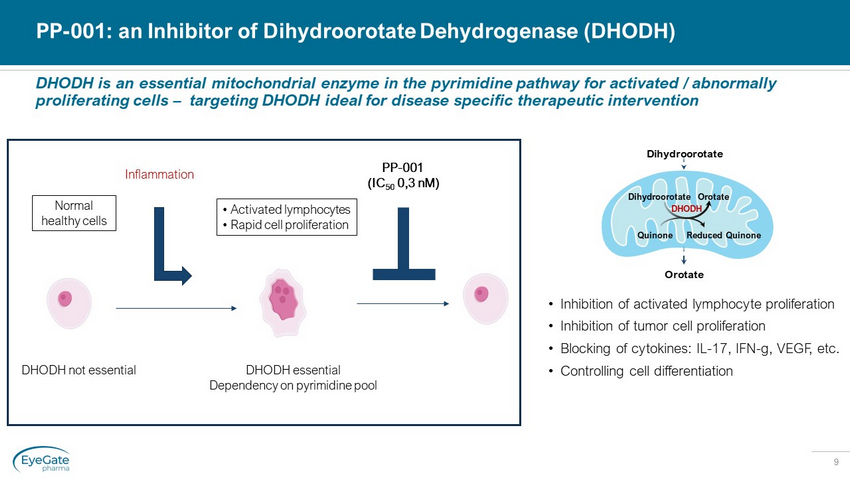
9 DHODH is an essential mitochondrial enzyme in the pyrimidine pathway for activated / abnormally proliferating cells – targeting DHODH ideal for disease specific therapeutic intervention PP - 001: an Inhibitor of Dihydroorotate Dehydrogenase (DHODH) • Inhibition of activated lymphocyte proliferation • Inhibition of tumor cell proliferation • Blocking of cytokines: IL - 17, IFN - g, VEGF, etc. • Controlling cell differentiation Dihydroorotate Orotate Orotate Dihydroorotate Quinone Reduced Quinone DHODH DHODH essential Dependency on pyrimidine pool DHODH not essential Normal healthy cells Inflammation • Activated lymphocytes • Rapid cell proliferation PP - 001 (IC 50 0,3 nM )
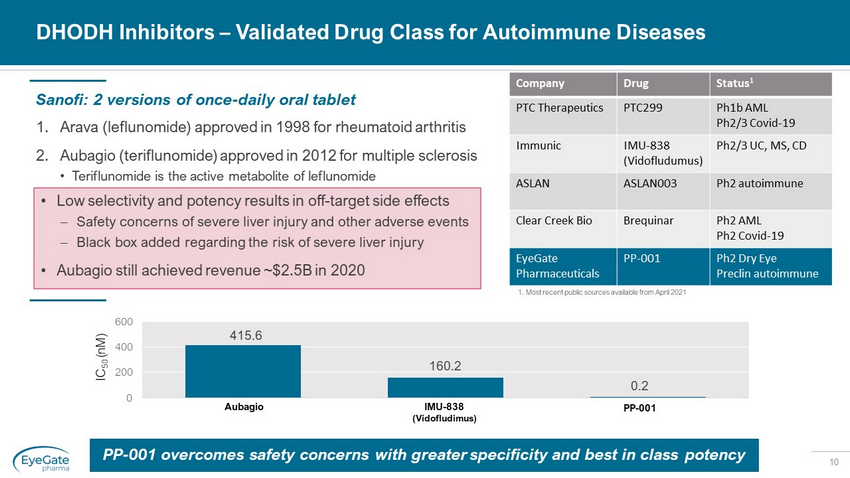
10 DHODH Inhibitors – Validated Drug Class for Autoimmune Diseases 1. Arava (leflunomide) approved in 1998 for rheumatoid arthritis 2. Aubagio (teriflunomide) approved in 2012 for multiple sclerosis • Teriflunomide is the active metabolite of leflunomide Sanofi: 2 versions of once - daily oral tablet PP - 001 overcomes safety concerns with greater specificity and best in class potency • Low selectivity and potency results in off - target side effects – Safety concerns of severe liver injury and other adverse events – Black box added regarding the risk of severe liver injury • Aubagio still achieved revenue ~$2.5B in 2020 1. Most recent public sources available from April 2021 Company Drug Status 1 PTC Therapeutics PTC299 Ph1b AML Ph2/3 Covid - 19 Immunic IMU - 838 ( Vidofludumus ) Ph2/3 UC, MS, CD ASLAN ASLAN003 Ph2 autoimmune Clear Creek Bio Brequinar Ph2 AML Ph2 Covid - 19 EyeGate Pharmaceuticals PP - 001 Ph2 Dry Eye Preclin autoimmune 415.6 160.2 0.2 0 200 400 600 Aubagio IMU - 838 (Vidofludimus) PP - 001 IC 50 ( nM )

11 PP - 001: A First - in - Class Drug for Ophthalmic Chronic Inflammation • Clinical development efforts focused in ophthalmology – High medical need for novel new immunomodulators/anti - inflammatories – Multiple diseases in the anterior and posterior regions of the eye offering substantial market opportunity • Initial safety and efficacy PoC studies 1. Dose - ascending study completed in 24 healthy volunteers 2. PoC efficacy study in 21 DED patients – Fully Enrolled
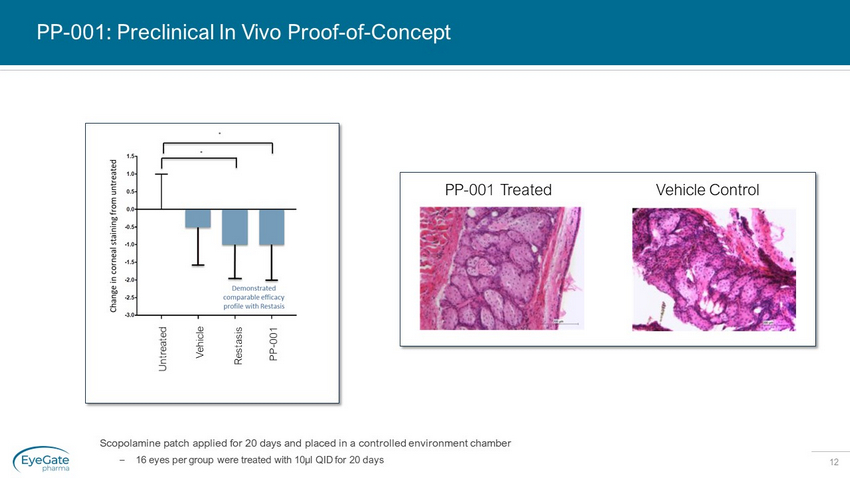
12 PP - 001: Preclinical In Vivo Proof - of - Concept Scopolamine patch applied for 20 days and placed in a controlled environment chamber – 16 eyes per group were treated with 10µl QID for 20 days Untreated Vehicle Restasis PP - 001 PP - 001 Treated Vehicle Control
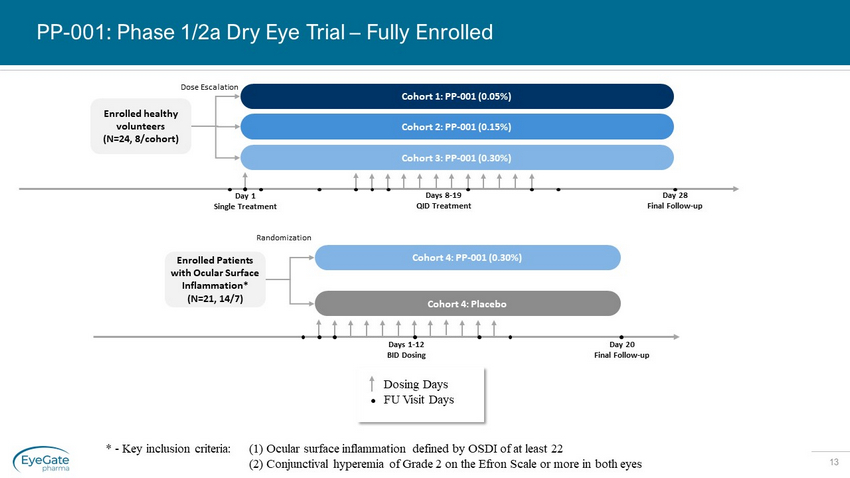
13 PP - 001: Phase 1/2a Dry Eye Trial – Fully Enrolled Enrolled healthy volunteer s (N=24, 8/cohort) Day 28 Final Follow - up Cohort 1: PP - 001 (0.05%) Cohort 2: PP - 001 (0.15%) Dose Escalation Day 1 Single Treatment Days 8 - 19 QID Treatment Cohort 3: PP - 001 (0.30%) * - Key inclusion criteria: (1) Ocular surface inflammation defined by OSDI of at least 22 (2) Conjunctival hyperemia of Grade 2 on the Efron Scale or more in both eyes Enrolled Patients with Ocular Surface Inflammation* (N=21, 14/7) Days 1 - 12 BID Dosing Day 20 Final Follow - up Cohort 4: PP - 001 (0.30%) Randomization Cohort 4: Placebo Dosing Days FU Visit Days

14 OBG Crosslinked Hyaluronic Acid
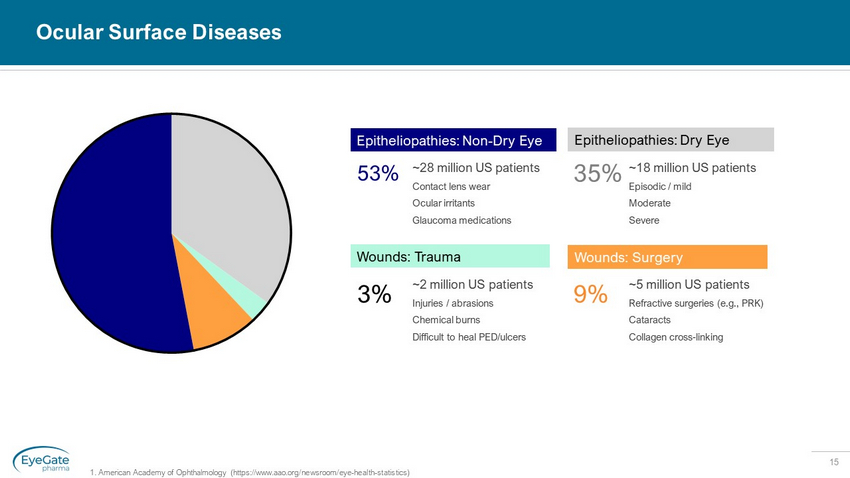
15 Ocular Surface Diseases ~2 million US patients Injuries / abrasions Chemical burns Difficult to heal PED/ulcers 3% ~28 million US patients Contact lens wear Ocular irritants Glaucoma medications 53% ~5 million US patients Refractive surgeries (e.g., PRK) Cataracts Collagen cross - linking 9% Wounds: Surgery ~18 million US patients Episodic / mild Moderate Severe 35% Epitheliopathies: Dry Eye Wounds: Trauma Epitheliopathies: Non - Dry Eye 1. American Academy of Ophthalmology (https://www.aao.org/newsroom/eye - health - statistics)
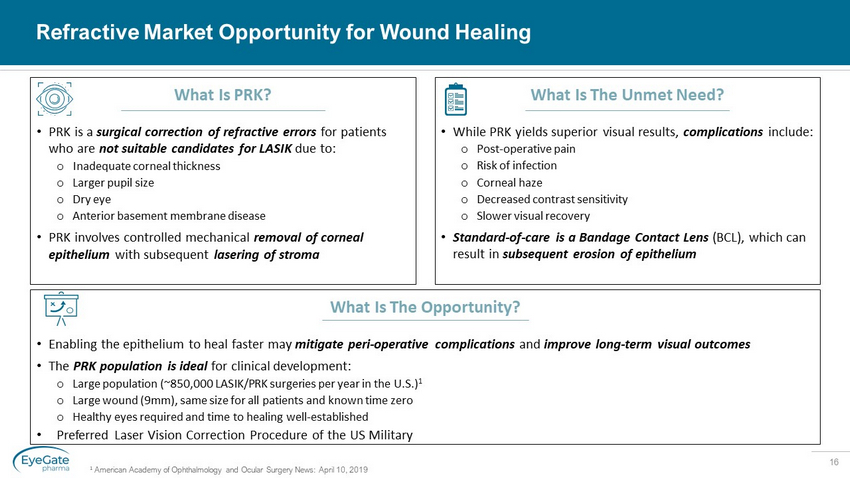
16 Refractive Market Opportunity for Wound Healing 1 American Academy of Ophthalmology and Ocular Surgery News: April 10, 2019 What Is PRK? • PRK is a surgical correction of refractive errors for patients who are not suitable candidates for LASIK due to: o Inadequate corneal thickness o Larger pupil size o Dry eye o Anterior basement membrane disease • PRK involves controlled mechanical removal of corneal epithelium with subsequent lasering of stroma What Is The Opportunity? • Enabling the epithelium to heal faster may mitigate peri - operative complications and improve long - term visual outcomes • The PRK population is ideal for clinical development: o Large population (~850,000 LASIK/PRK surgeries per year in the U.S.) 1 o Large wound (9mm), same size for all patients and known time zero o Healthy eyes required and time to healing well - established • Preferred Laser Vision Correction Procedure of the US Military What Is The Unmet Need? • While PRK yields superior visual results, complications include: o Post - operative pain o Risk of infection o Corneal haze o Decreased contrast sensitivity o Slower visual recovery • Standard - of - care is a Bandage Contact Lens (BCL), which can result in subsequent erosion of epithelium
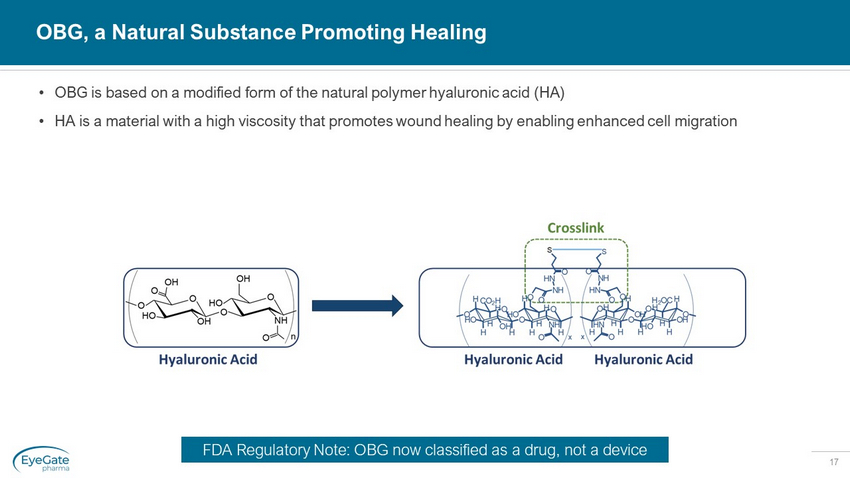
17 • OBG is based on a modified form of the natural polymer hyaluronic acid (HA) • HA is a material with a high viscosity that promotes wound healing by enabling enhanced cell migration OBG, a Natural Substance Promoting Healing FDA Regulatory Note: OBG now classified as a drug, not a device

18 Covalent Crosslinking Creates Unique Attributes Ideal for Ocular Surface • Improved Product Stability • Longer retention on the ocular surface over non - crosslinked HA (2 hours vs minutes) • Higher shear - thinning properties: – Able to achieve concentrations up to 7.5x current products (0.75% vs. others at 0.1 - 0.4%) – Decreased viscosity during blinking = no blurred vision • 5 studies completed (3 PRK surgery and 2 dry eye) and ~400 eyes have been treated with OBG – Strong Safety and Efficacy profile Demonstrated superiority of accelerated wound healing over a bandage contact lens in a late - stage clinical trial
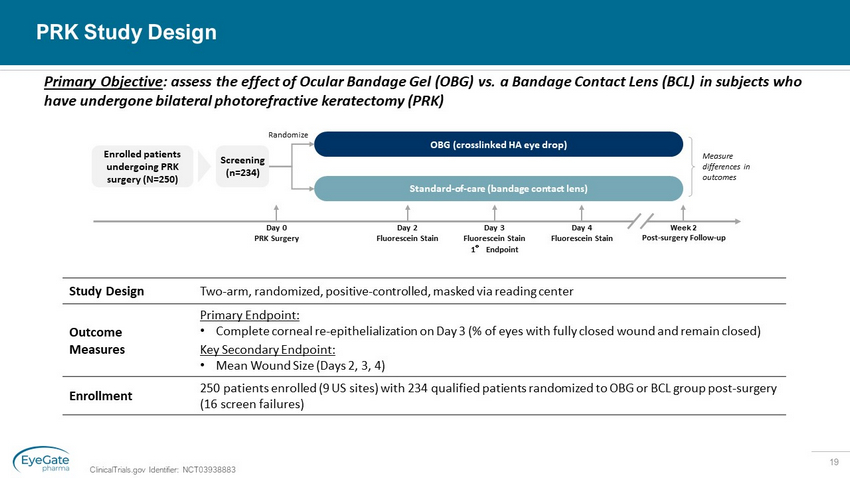
19 PRK Study Design ClinicalTrials.gov Identifier: NCT03938883 Enrolled patients undergoing PRK surgery (N=250) Day 0 PRK Surgery Week 2 Post - surgery Follow - up Measure differences in outcomes Screening (n=234) OBG (crosslinked HA eye drop) Standard - of - care (bandage contact lens) Randomize Day 2 Fluorescein Stain Day 3 F luorescein Stain 1 ° Endpoint Day 4 F luorescein Stain Study Design Two - arm, randomized, positive - controlled, masked via reading center Outcome Measures Primary Endpoint: • Complete corneal re - epithelialization on Day 3 (% of eyes with fully closed wound and remain closed) Key Secondary Endpoint: • Mean Wound Size (Days 2, 3, 4) Enrollment 250 patients enrolled (9 US sites) with 234 qualified patients randomized to OBG or BCL group post - surgery (16 screen failures) Primary Objective : assess the effect of Ocular Bandage Gel (OBG) vs. a Bandage Contact Lens (BCL) in subjects who have undergone bilateral photorefractive keratectomy (PRK)
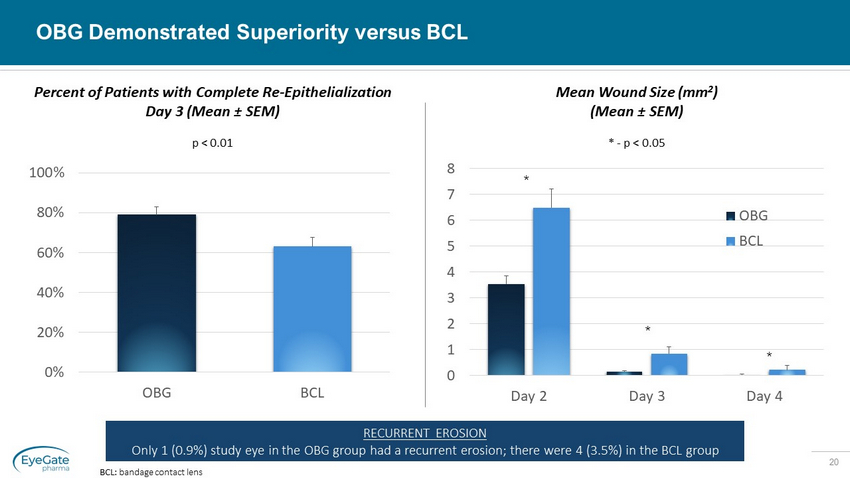
20 OBG Demonstrated Superiority versus BCL BCL: bandage contact lens BCL BCL 0% 20% 40% 60% 80% 100% OBG BCL Percent of Patients with Complete Re - Epithelialization Day 3 (Mean ± SEM) p < 0.01 RECURRENT EROSION Only 1 (0.9%) study eye in the OBG group had a recurrent erosion; there were 4 (3.5%) in the BCL group OBG OBG 0 1 2 3 4 5 6 7 8 Day 2 Day 3 Day 4 OBG BCL Mean Wound Size (mm 2 ) (Mean ± SEM) * - p < 0.05 * * *

21 B - 203 (BENAQ) Molecular Photoswitch * * - Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation
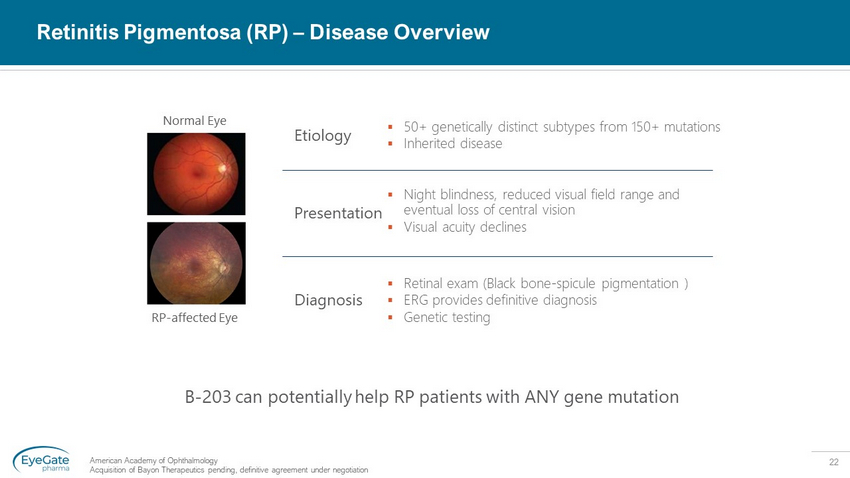
22 Retinitis Pigmentosa (RP) – Disease Overview American Academy of Ophthalmology Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation ▪ 50+ genetically distinct subtypes from 150+ mutations ▪ Inherited disease Etiology ▪ Night blindness, reduced visual field range and eventual loss of central vision ▪ Visual acuity declines Presentation ▪ Retinal exam (Black bone - spicule pigmentation ) ▪ ERG provides definitive diagnosis ▪ Genetic testing Diagnosis Normal Eye RP - affected Eye B - 203 can potentially help RP patients with ANY gene mutation
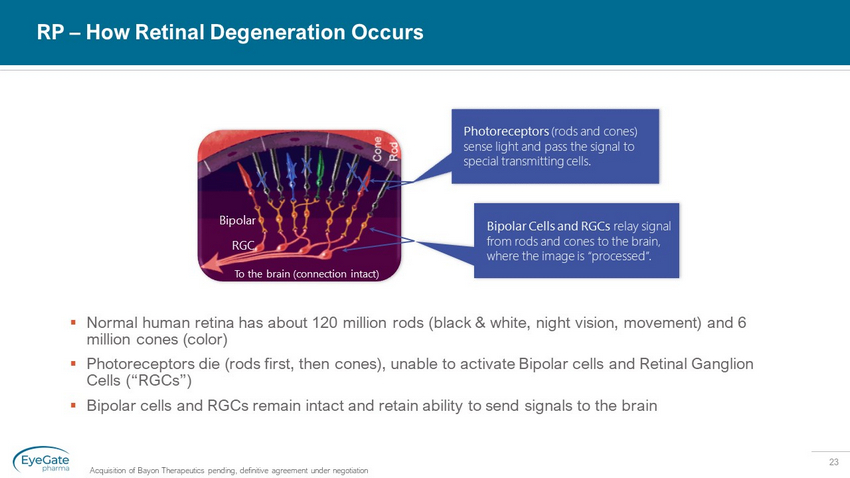
23 RP – How Retinal Degeneration Occurs Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation ▪ Normal human retina has about 120 million rods (black & white, night vision, movement) and 6 million cones (color) ▪ Photoreceptors die (rods first, then cones), unable to activate Bipolar cells and Retinal Ganglion Cells (“RGCs”) ▪ Bipolar cells and RGCs remain intact and retain ability to send signals to the brain To the brain (connection intact) Photoreceptors (rods and cones) sense light and pass the signal to special transmitting cells. Bipolar Cells and RGCs relay signal from rods and cones to the brain, where the image is “processed”. Bipolar RGC X X X X X
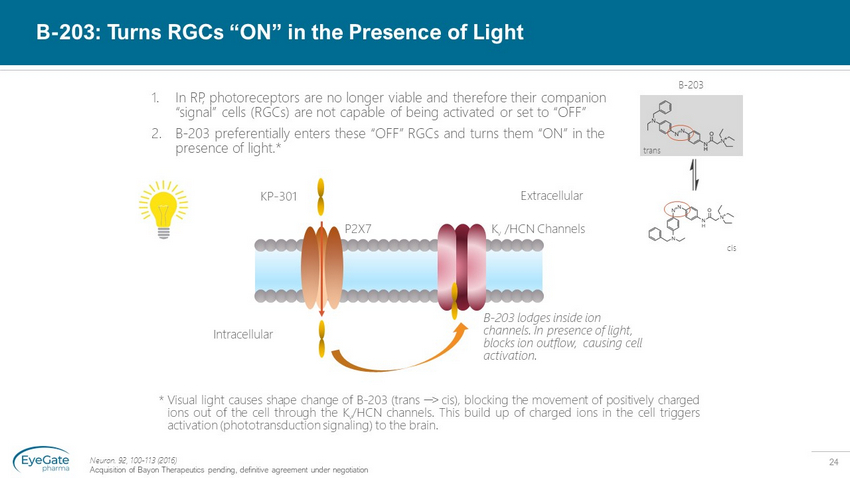
24 B - 203: Turns RGCs “ON” in the Presence of Light Neuron. 92, 100 - 113 (2016) Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation 1. In RP, photoreceptors are no longer viable and therefore their companion “signal” cells (RGCs) are not capable of being activated or set to “OFF” 2. B - 203 preferentially enters these “OFF” RGCs and turns them “ON” in the presence of light.* P2X7 BENAQ K v Channel Intracellular Extracellular KP - 301 K v /HCN Channels P2X7 Extracellular Intracellular trans cis B - 203 * Visual light causes shape change of B - 203 (trans ፨ ˃ cis), blocking the movement of positively charged ions out of the cell through the K v /HCN channels . This build up of charged ions in the cell triggers activation (phototransduction signaling) to the brain . B - 203 lodges inside ion channels. In presence of light, blocks ion outflow, causing cell activation.
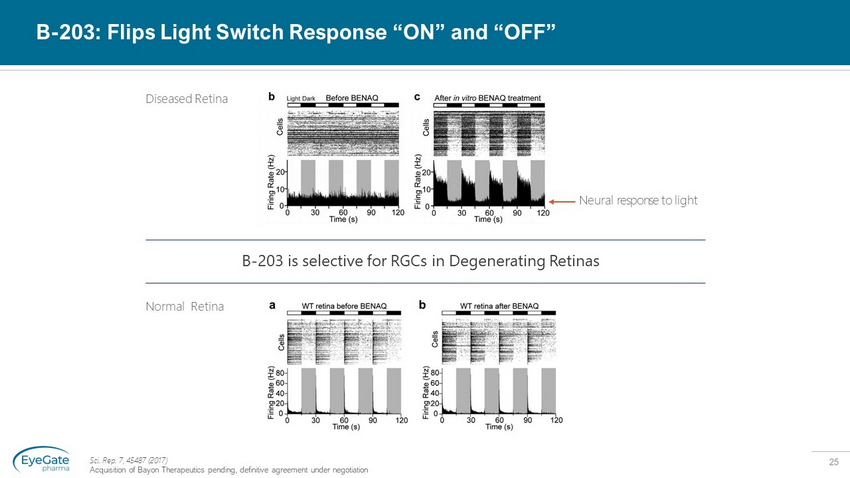
25 B - 203: Flips Light Switch Response “ON” and “OFF” Sci. Rep. 7, 45487 (2017) Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation Neural response to light B - 203 is selective for RGCs in Degenerating Retinas Normal Retina Diseased Retina
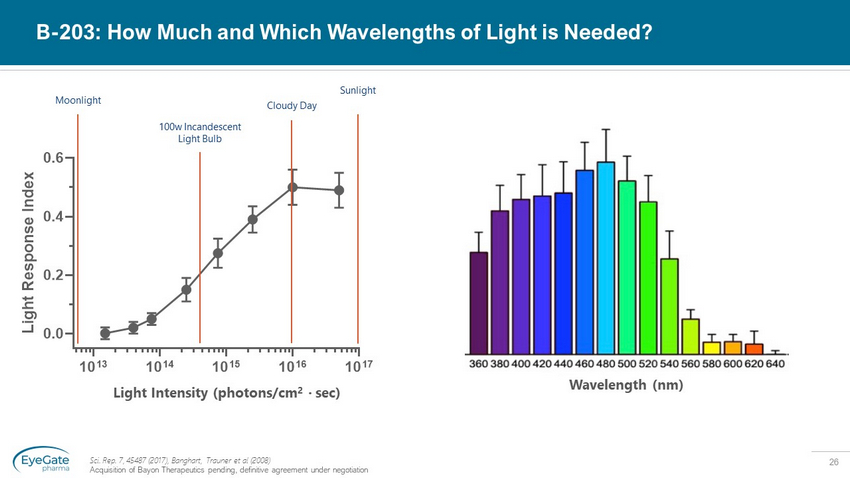
26 10 13 10 14 10 15 10 16 10 17 0.0 0.2 0.4 0.6 Light Intensity (photons/cm 2sec) L i g h t R e s p o n s e I n d e x Moonlight 100w Incandescent Light Bulb Cloudy Day Sunlight Light Intensity (photons/cm 2 · sec) B - 203: How Much and Which Wavelengths of Light is Needed? Sci. Rep. 7, 45487 (2017), Banghart, Trauner et al (2008) Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation Wavelength (nm)
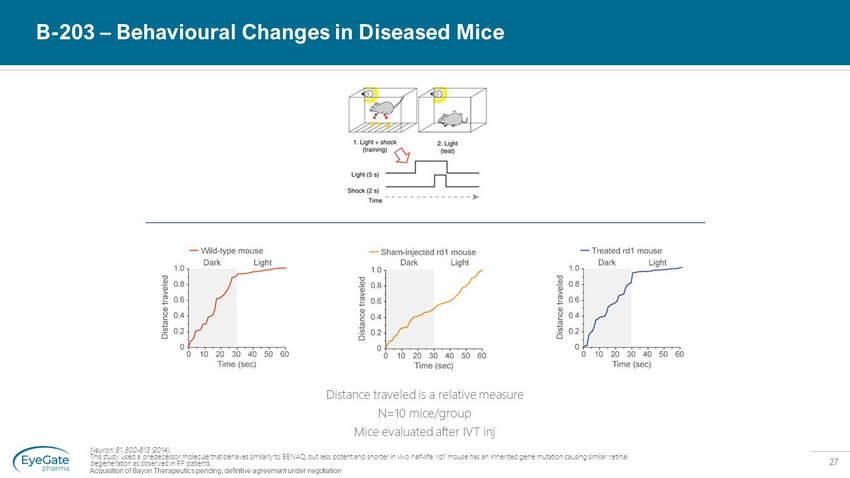
27 B - 203 – Behavioural Changes in Diseased Mice Neuron. 81, 800 - 813 (2014). This study used a predecessor molecule that behaves similarly to BENAQ, but less potent and shorter in vivo half - life. rd1 mouse has an inherited gene mutation causing similar retinal degeneration as observed in RP patients. Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation Distance traveled is a relative measure N=10 mice/group Mice evaluated after IVT inj
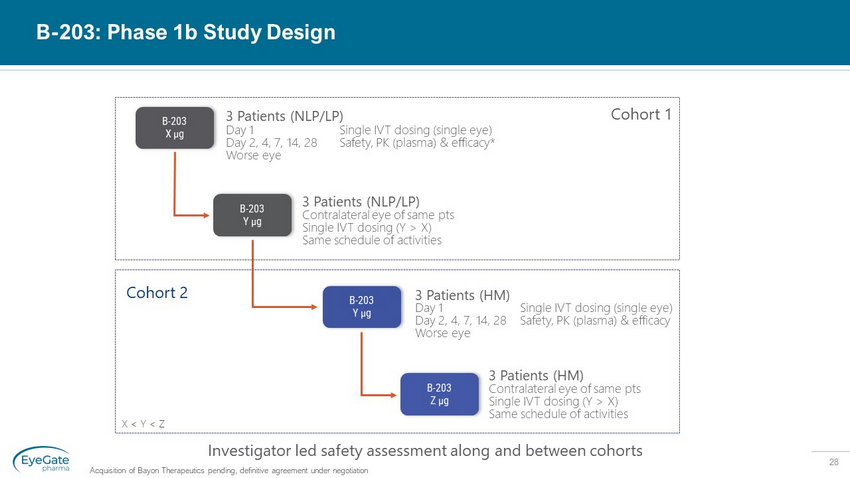
28 B - 203 X µg B - 203 Y µg B - 203 Y µg B - 203 Z µg 3 Patients (NLP/LP) Contralateral eye of same pts Single IVT dosing (Y > X) Same schedule of activities 3 Patients (HM) Investigator led safety assessment along and between cohorts 3 Patients (NLP/LP) Cohort 1 Cohort 2 X < Y < Z Day 1 Day 2, 4, 7, 14, 28 Worse eye Single IVT dosing (single eye) Safety, PK (plasma) & efficacy* Day 1 Day 2, 4, 7, 14, 28 Worse eye Single IVT dosing (single eye) Safety, PK (plasma) & efficacy 3 Patients (HM) Contralateral eye of same pts Single IVT dosing (Y > X) Same schedule of activities B - 203: Phase 1b Study Design Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation
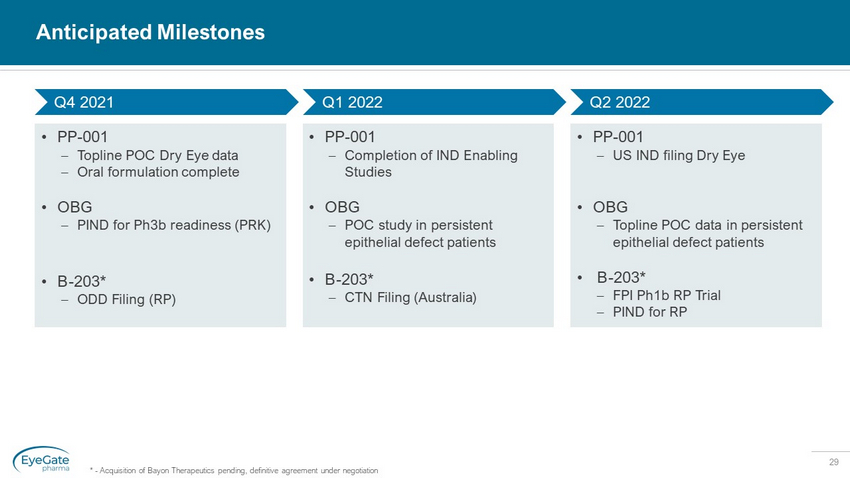
29 Anticipated Milestones • PP - 001 – Topline POC Dry Eye data – Oral formulation complete • OBG – PIND for Ph3b readiness (PRK) • B - 203* – ODD Filing (RP) • PP - 001 – US IND filing Dry Eye • OBG – Topline POC data in persistent epithelial defect patients • B - 203* – FPI Ph1b RP Trial – PIND for RP • PP - 001 – Completion of IND Enabling Studies • OBG – POC study in persistent epithelial defect patients • B - 203* – CTN Filing (Australia) Q4 2021 Q1 2022 Q2 2022 * - Acquisition of Bayon Therapeutics pending, definitive agreement under negotiation

Sept 2021 THANK YOU