EXHIBIT 99.1
Published on May 27, 2021
Exhibit 99.1

Treating inflammatory and immune diseases May 2021

2 Forward Looking Statements Some of the statements are “forward - looking” and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995 . These “forward - looking” statements include statements relating to, among other things, the commercialization efforts and other regulatory or marketing approval efforts pertaining to EyeGate’s products, including EyeGate’s PP - 001 and OBG products, as well as the success thereof, with such approvals or success may not be obtained or achieved on a timely basis or at all . These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this press release, including, among other things, certain risk factors described under the heading “Risk Factors” contained in EyeGate’s Annual Report on Form 10 - K filed with the SEC on March 25 , 2021 or described in EyeGate’s other public filings . EyeGate’s results may also be affected by factors of which EyeGate is not currently aware . The forward - looking statements in this press release speak only as of the date of this press release . EyeGate expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions or circumstances on which any such statement is based .
3 Investment Highlights • PP - 001 completed safety studies for eye drop and intravitreal injection formulations • OBG completed multiple studies for wound healing and dry eye Clinical Stage • PP - 001 eye drops for dry eye and allergic conjunctivitis • OBG re - classified from a device to a drug for dry eye and ocular wounds Deepening Ocular Surface Franchise • PP - 001 already formulated for intravenous delivery; oral formulation in process • I.V. and oral formulations applicable for broad systemic indications with tremendous potential • I.V. formulation for oncology and oral formulation for autoimmune diseases and oncology Expansion into Systemic Diseases • PP - 001 – 4 th generation small - molecule inhibitor of Dihydroorotate Dehydrogenase (DHODH) • Best - in - class picomolar potency to overcome off - target side effects of Aubagio and Arava Novel Molecule in Proven Class • EyeGate pipeline for ophthalmology transformed by Panoptes acquisition • PP - 001 provides additional indications in ophthalmology and systemic diseases Merger Creates Expanded Pipeline EYEG NASDAQ listed

4 Company Overview PP - 001: fourth generation small - molecule inhibitor of Dihydroorotate Dehydrogenase (DHODH) – Validated class, first approved for RA (Sanofi - Arava) and then approved for MS (Sanofi - Aubagio) – Best - in - class with picomolar potency to overcome off - target side effects of prior generations – Primary mode - of - action applicable in autoimmune, oncology and viral infections – Initial formulations for ophthalmology with clinical safety studies completed – IV and oral formulations under development for broad systemic indications with significant potential EyeGate is a clinical - stage company with two unique platforms: PP - 001 and OBG OBG: modified form of the natural polymer Hyaluronic Acid (HA) – Formulated as an eye drop that promotes wound healing and provides lubrication – Clinical studies completed demonstrating accelerated wound healing in PRK patients and treatment of dry eye – Recent reclassification from device to drug division of FDA

5 Transformed from a Device Company to a Drug Pharma Company – OBG ocular surface franchise : • U.S. IND submission and initiation of Phase 2 dry eye study planned in Q4:21 • Proof - of - concept study for persistent epithelial defects expected to initiate in Q4:21 – PP - 001 ocular surface franchise: • Proof - of - concept study in Austria for dry eye patients expected to initiate in Q3:21 • U.S. IND submission planned in Q4:21 – PP - 001 for systemic indications: • Phase 1 healthy volunteer study in Austria planned to initiate in Q4:21 With OBG now being developed as a drug and the recent addition of PP - 001 there are multiple meaningful inflection points over the next 12 to 18 months
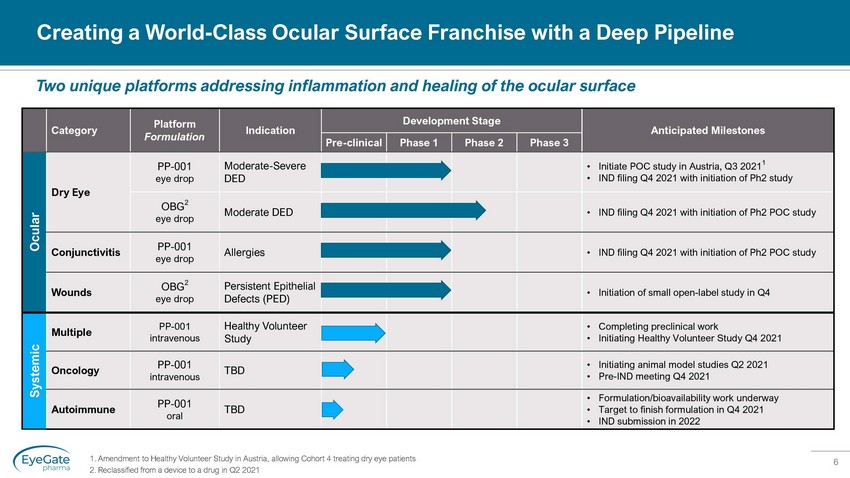
6 Creating a World - Class Ocular Surface Franchise with a Deep Pipeline 1. Amendment to Healthy Volunteer Study in Austria, allowing Cohort 4 treating dry eye patients 2. Reclassified from a device to a drug in Q2 2021 Two unique platforms addressing inflammation and healing of the ocular surface Systemic Multiple PP - 001 intravenous Healthy Volunteer Study • Completing preclinical work • Initiating Healthy Volunteer Study Q4 2021 Oncology PP - 001 intravenous TBD • Initiating animal model studies Q2 2021 • Pre - IND meeting Q4 2021 Autoimmune PP - 001 oral TBD • Formulation/bioavailability work underway • Target to finish formulation in Q4 2021 • IND submission in 2022 Category Platform Formulation Indication Development Stage Anticipated Milestones Pre - clinical Phase 1 Phase 2 Phase 3 Ocular Dry Eye PP - 001 eye drop Moderate - Severe DED • Initiate POC study in Austria, Q3 2021 1 • IND filing Q4 2021 with initiation of Ph2 study OBG 2 eye drop Moderate DED • IND filing Q4 2021 with initiation of Ph2 POC study Conjunctivitis PP - 001 eye drop Allergies • IND filing Q4 2021 with initiation of Ph2 POC study Wounds OBG 2 eye drop Persistent Epithelial Defects (PED) • Initiation of small open - label study in Q4

DHODH Inhibitor PP - 001
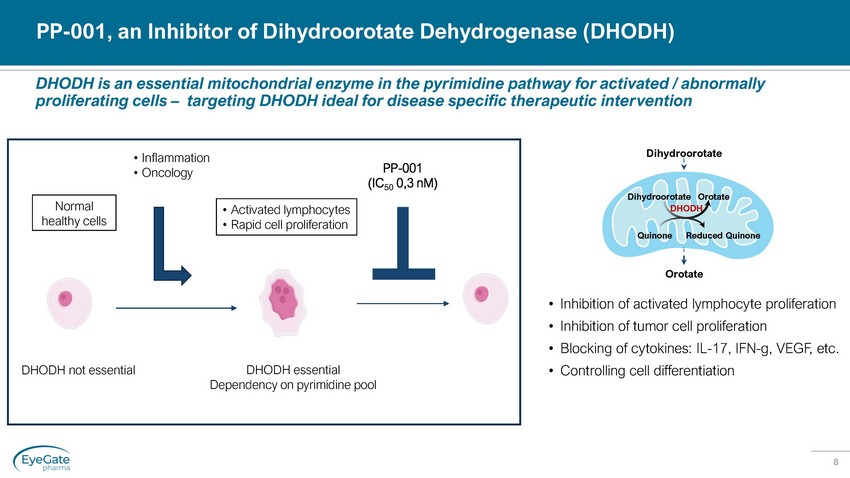
8 DHODH is an essential mitochondrial enzyme in the pyrimidine pathway for activated / abnormally proliferating cells – targeting DHODH ideal for disease specific therapeutic intervention PP - 001, an Inhibitor of Dihydroorotate Dehydrogenase (DHODH) • Inhibition of activated lymphocyte proliferation • Inhibition of tumor cell proliferation • Blocking of cytokines: IL - 17, IFN - g, VEGF, etc. • Controlling cell differentiation Dihydroorotate Orotate Orotate Dihydroorotate Quinone Reduced Quinone DHODH DHODH essential Dependency on pyrimidine pool DHODH not essential Normal healthy cells • Inflammation • Oncology • Activated lymphocytes • Rapid cell proliferation PP - 001 (IC 50 0,3 nM )
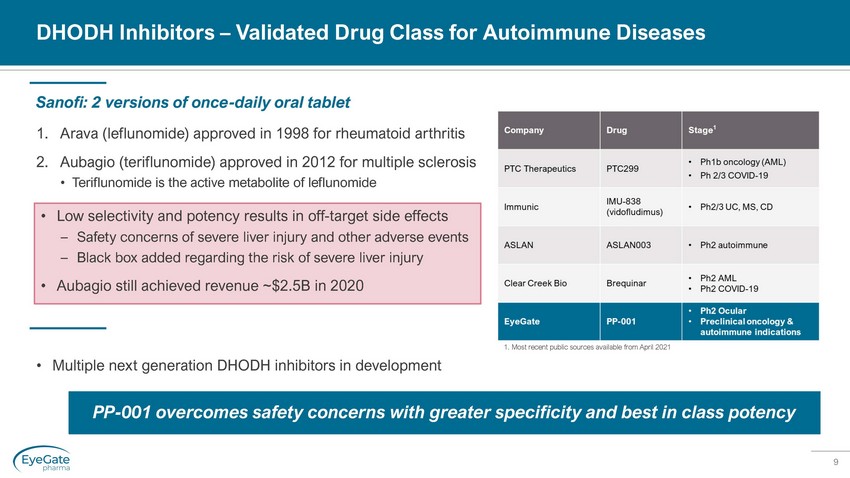
9 DHODH Inhibitors – Validated Drug Class for Autoimmune Diseases 1. Arava (leflunomide) approved in 1998 for rheumatoid arthritis 2. Aubagio (teriflunomide) approved in 2012 for multiple sclerosis • Teriflunomide is the active metabolite of leflunomide Sanofi: 2 versions of once - daily oral tablet PP - 001 overcomes safety concerns with greater specificity and best in class potency • Low selectivity and potency results in off - target side effects – Safety concerns of severe liver injury and other adverse events – Black box added regarding the risk of severe liver injury • Aubagio still achieved revenue ~$2.5B in 2020 • Multiple next generation DHODH inhibitors in development 1. Most recent public sources available from April 2021
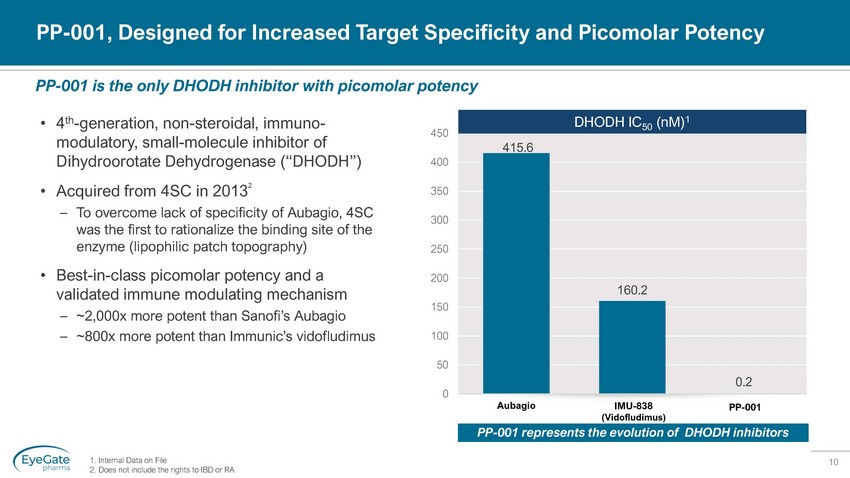
10 415.6 160.2 0.2 0 50 100 150 200 250 300 350 400 450 PP - 001, Designed for Increased Target Specificity and Picomolar Potency • 4 th - generation, non - steroidal, immuno - modulatory, small - molecule inhibitor of Dihydroorotate Dehydrogenase (“DHODH”) • Acquired from 4SC in 2013 2 – To overcome lack of specificity of Aubagio, 4SC was the first to rationalize the binding site of the enzyme (lipophilic patch topography) • Best - in - class picomolar potency and a validated immune modulating mechanism – ~2,000x more potent than Sanofi’s Aubagio – ~800x more potent than Immunic’s vidofludimus PP - 001 is the only DHODH inhibitor with picomolar potency DHODH IC 50 ( nM ) 1 Aubagio IMU - 838 (Vidofludimus) PP - 001 1. Internal Data on File 2. Does not include the rights to IBD or RA PP - 001 represents the evolution of DHODH inhibitors

11 PP - 001 is a First - in - Class Drug for Ophthalmology Indications • First clinical development efforts focused in ophthalmology – High medical need for novel new immunomodulators/anti - inflammatories – Multiple diseases in the anterior and posterior regions of the eye offering substantial market opportunity • Initial safety studies completed with two different ocular formulations 1. Eye drop: Dose - ascending study completed in 24 healthy volunteers (Austria) – Can now go straight into Phase 2 studies in multiple indications (preparing for meeting with FDA) 2. Intravitreal Injection: Dose - ascending study completed in 12 patients with chronic non - infectious uveitis (Europe) – Future clinical development for retinal diseases will require a new formulation
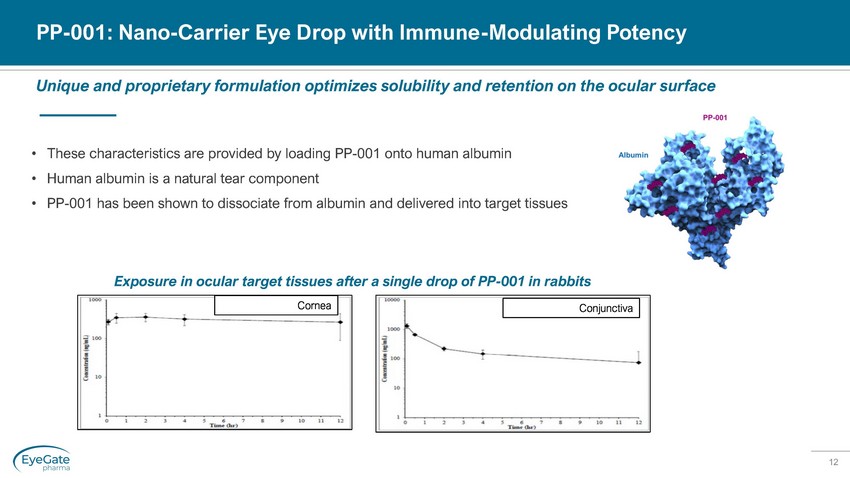
12 PP - 001: Nano - Carrier Eye Drop with Immune - Modulating Potency Unique and proprietary formulation optimizes solubility and retention on the ocular surface • These characteristics are provided by loading PP - 001 onto human albumin • Human albumin is a natural tear component • PP - 001 has been shown to dissociate from albumin and delivered into target tissues Conjunctiva Cornea

13 Phase 1 Dose - Ascending Study Completed • Double - masked, placebo - controlled, randomized, dose escalating study in 24 healthy volunteers (Austria) • Purpose: safety, tolerability, and systemic PK assessment • Results: ✓ Established safety and no SAEs ✓ Excellent tolerability with no burning or stinging effect on instillation ✓ No systemic exposure • Next Steps: – POC study in dry eye patients in Austria – Open IND and initiate Ph 2 clinical studies in patients with dry eye and patients with allergic conjunctivitis PP - 001 Eye Drop Safety Study in Healthy Volunteers
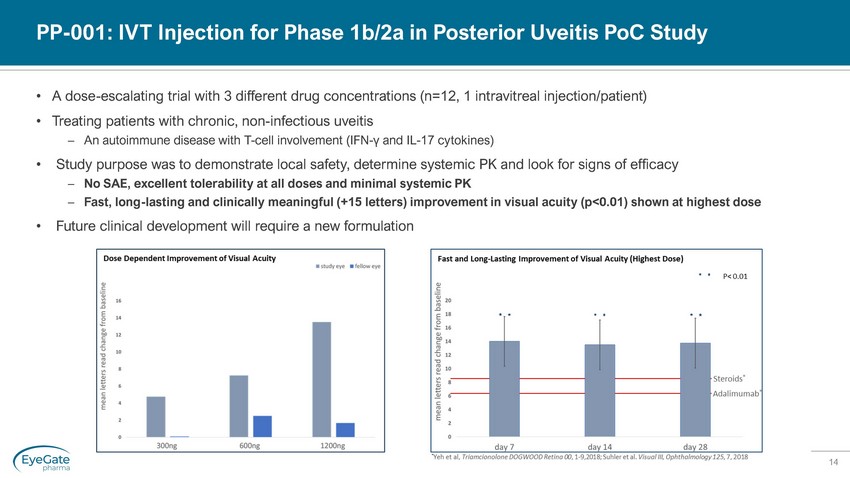
14 PP - 001: IVT Injection for Phase 1b/2a in Posterior Uveitis PoC Study • A dose - escalating trial with 3 different drug concentrations (n=12, 1 intravitreal injection/patient) • Treating patients with chronic, non - infectious uveitis – An autoimmune disease with T - cell involvement (IFN - γ and IL - 17 cytokines) • Study purpose was to demonstrate local safety, determine systemic PK and look for signs of efficacy – No SAE, excellent tolerability at all doses and minimal systemic PK – Fast, long - lasting and clinically meaningful (+15 letters) improvement in visual acuity (p<0.01) shown at highest dose • Future clinical development will require a new formulation
15 DHODHi Mechanism of Action Applicable Across Several Categories • Oral formulation underway • Targeting IND H1 2022 Oral PP - 001 • IV formulation completed – Preclinical tox ongoing – HVS targeted for Q4 2021 (Austria) – Pre - IND meeting by year - end IV PP - 001 • Will assess post the HV study • Opportunistic PP - 001 Large opportunity for PP - 001 in systemic diseases: potency (~2,000X Aubagio) Autoimmune/Anti - inflammatory – The class is well validated for several autoimmune diseases: RA and MS – Even with black box warning Aubagio achieved ~$2.5B 1 in sales in 2020 Oncology – Cancer cells rewire their metabolism to enhance de novo pathway – DHODH is essential for the survival of several tumor types, e.g., lung breast, AML (regulates myeloid differentiation) and others Viral Infections – Inhibits human proteins interacting with virus rather than the virus itself – As a host - targeting antiviral (HTA) can target a broad spectrum of viruses 1. Form 20 - F 2020: €2,045 and using 1.20 to 1 exchange rate

16 PP - 001: Preparing for the Treatment of Systemic Disease Intravenous Formulation • Grant received from Austrian government in 2020 for development of IV formulation – IV formulation of anti - inflammatory required to treat late - stage (intubated) COVID patients – Formulation work completed and preclinical tox work underway – Next step is Ph1 SAD/MAD volunteer safety study in Austria (targeting initiation Q4 2021) – Medical need for COVID will be determined post completion of safety study – Will pivot clinical work to treatment of cancer patients (pre - IND meeting by year - end 2021) • Tumor cell line screening completed, moving into animal models Oral Formulation • An oral formulation provides access to very large chronic markets (e.g., autoimmune and oncology) – Formulation work has been initiated (i.e., salt/co - crystallization and bioavailability work) – IND planned for 2022
17 PP - 001: Oncology Development Strategy PP - 001 as the most potent DHODHi showed in vitro activity in solid and hematological tumors 1. Completed in vitro efficacy – ~200 tumor cell lines screened Results • Inhibits proliferation of 10 - 15% of solid and hematological tumor cell lines at sub - micromolar concentrations ✓ no general toxicities but selective inhibition of cell proliferation as predicted ✓ confirmed that efficacy is dependent on DHODH inhibition 2. Animal experiments initiated 3. Pre - IND meeting / IND submission – Pre - IND meeting early Q4 – IND submission by year - end 2021 – In vivo data expected late Q3/early Q4

18 OBG Crosslinked Hyaluronic Acid Eyedrop for Dry Eye
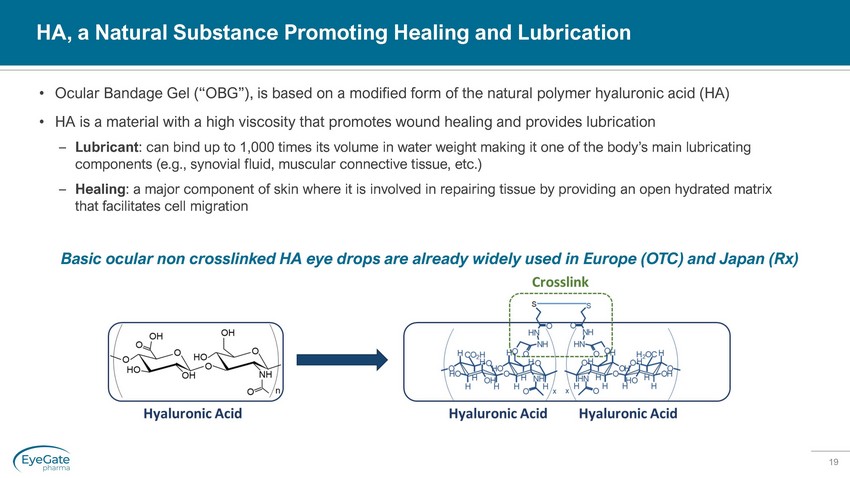
19 HA, a Natural Substance Promoting Healing and Lubrication • Ocular Bandage Gel (“OBG”), is based on a modified form of the natural polymer hyaluronic acid (HA) • HA is a material with a high viscosity that promotes wound healing and provides lubrication – Lubricant : can bind up to 1,000 times its volume in water weight making it one of the body’s main lubricating components (e.g., synovial fluid, muscular connective tissue, etc.) – Healing : a major component of skin where it is involved in repairing tissue by providing an open hydrated matrix that facilitates cell migration

20 Covalent Crosslinking Creates Unique Attributes Ideal for Ocular Surface • Prevents degradation, predominately excreted in crosslinked form • Much longer retention on the ocular surface over non - crosslinked HA (2 hours vs minutes) • Higher shear - thinning properties: – Able to achieve concentrations up to 7.5x current products (0.75% vs. others at 0.1 - 0.4%) – Also results in decreased viscosity during blinking which means no blurred vision • Will be the only Rx eye drop in the U.S. with HA and only eye drop (OTC) with HA as the sole “active” ingredient – The only known covalently crosslinked HA eye drop – Some current OTC products have HA, but an “inactive” ingredient • 5 studies completed (3 PRK surgery and 2 dry eye) and ~400 eyes have been treated with OBG • Completed pre - IND meeting in March 2021, preparing to file IND Demonstrated superiority of accelerated wound healing over a bandage contact lens in a pivotal study
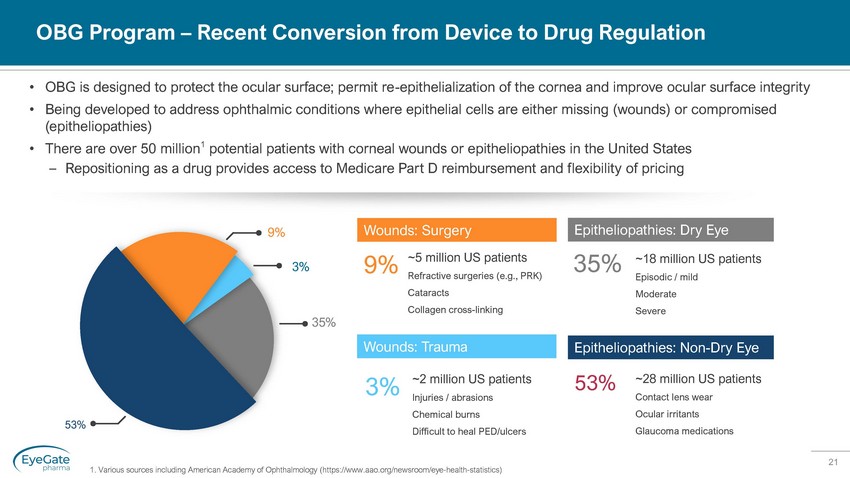
21 OBG Program – Recent Conversion from Device to Drug Regulation • OBG is designed to protect the ocular surface; permit re - epithelialization of the cornea and improve ocular surface integrity • Being developed to address ophthalmic conditions where epithelial cells are either missing (wounds) or compromised (epitheliopathies) • There are over 50 million 1 potential patients with corneal wounds or epitheliopathies in the United States – Repositioning as a drug provides access to Medicare Part D reimbursement and flexibility of pricing ~2 million US patients Injuries / abrasions Chemical burns Difficult to heal PED/ulcers 3% ~28 million US patients Contact lens wear Ocular irritants Glaucoma medications 53% ~5 million US patients Refractive surgeries (e.g., PRK) Cataracts Collagen cross - linking 9% Wounds: Surgery ~18 million US patients Episodic / mild Moderate Severe 35% Epitheliopathies: Dry Eye Wounds: Trauma Epitheliopathies: Non - Dry Eye 9% 3% 35% 1. Various sources including American Academy of Ophthalmology (https://www.aao.org/newsroom/eye - health - statistics)
22 PP - 001 AND OBG TREATING OCULAR SURFACE INDICATIONS • Dry eye: PP - 001 and OBG • Allergic conjunctivitis: PP - 001 • Ocular surface wounds: OBG
23 Dry Eye: Opportunity • A multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film – Inflammation is the common denominator of pathogenesis • Significantly affects the quality of life – Chronicity, pain and irritation, blindness in severe form • Tens of millions worldwide – 9 million 1 people in U.S. have the moderate/severe form of DED • No definitive treatment that works in most patients: only ~1.6 out of 9 million patients are being treated – Cyclosporine 0.05% (Restasis®, Allergan - 2020 US sales $1.3 billion 2 ) – Lifitegrast 5% (Xiidra®, Shire - 2020 US sales of $376M 3 ) – Steroids • To date only anti - inflammatories have been approved by FDA – Will require multiple mechanisms to satisfy patient population – Xiidra’s introduction in 2016 helped increase overall prescribing 4 • Rx’s went from ~3mn to ~4mn per annum (only 1.4mn unique patients in 2020) 1. Global Data's Dry Eye Syndrome Global Drug Forecast and Market Analysis to 2026: Published June 2018 2. Based on Q4 2020 run rate reported by Abbvie 3. 2020 sales reported by Novartis 4. Symphony Health – OIS presentation 2020
24 Dry Eye: T - Cell Mediated Disease • Desiccating stress (tear film instability and hyperosmolarity) leads to compromised corneal epithelial cells and increased expression of pro - inflammatory cytokines on the ocular surface • Inflammatory cytokines lead to APC activation – Migrate to lymph nodes • T cell activation in draining lymph nodes – Differentiation into Th1 and Th17 effector cells – Additional pro - inflammatory cytokines (IFN - γ and IL - 17) • Effector cells migrate to ocular surface • TH17 - mediated response is potentially responsible for meibomian glands (MG) obstruction 1 1. Reyes et al, Sci. Transl. Med, 25 Jul 2018: Vol. 10, Issue 451, eaas9164
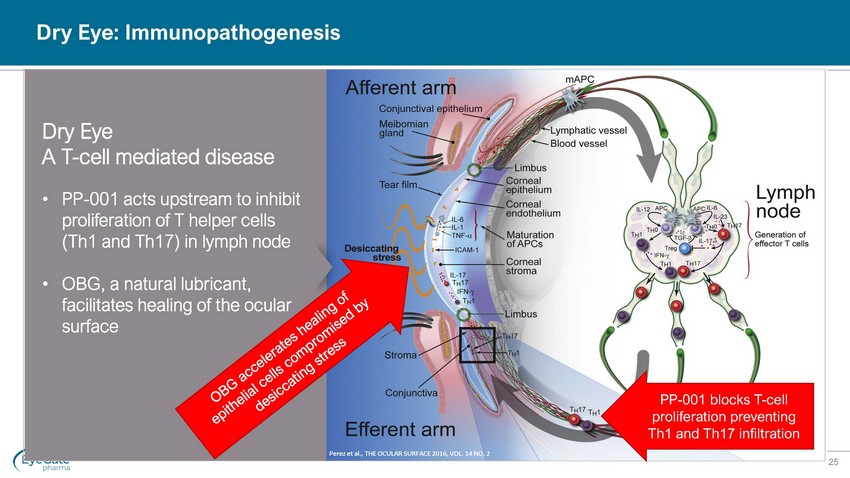
25 Dry Eye: Immunopathogenesis PP - 001 blocks T - cell proliferation preventing Th1 and Th17 infiltration Dry Eye A T - cell mediated disease • PP - 001 acts upstream to inhibit proliferation of T helper cells (Th1 and Th17) in lymph node • OBG, a natural lubricant, facilitates healing of the ocular surface Perez et al., THE OCULAR SURFACE 2016, VOL. 14 NO. 2
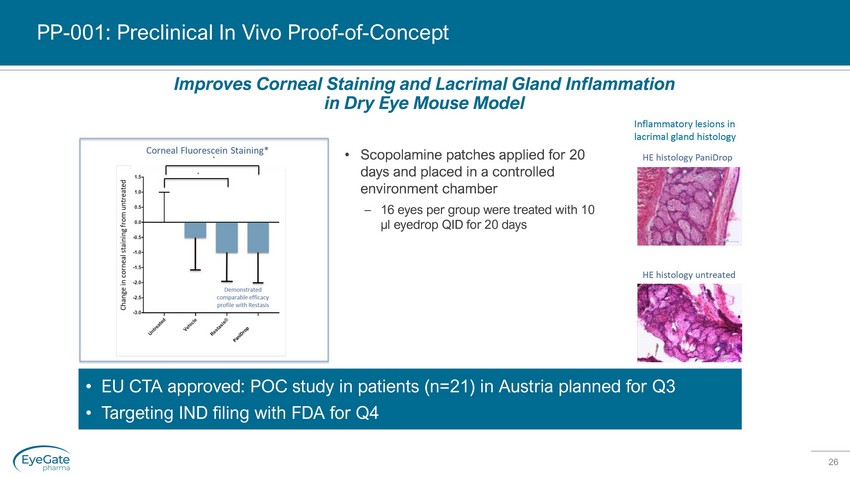
26 PP - 001: Preclinical In Vivo Proof - of - Concept • Scopolamine patches applied for 20 days and placed in a controlled environment chamber – 16 eyes per group were treated with 10 µl eyedrop QID for 20 days • EU CTA approved: POC study in patients (n=21) in Austria planned for Q3 • Targeting IND filing with FDA for Q4
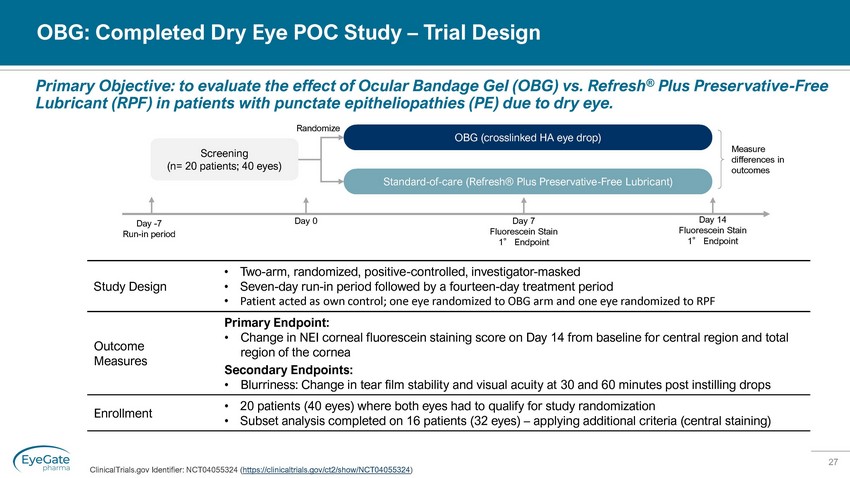
27 OBG: Completed Dry Eye POC Study – Trial Design ClinicalTrials.gov Identifier: NCT04055324 ( https://clinicaltrials.gov/ct2/show/NCT04055324 ) Primary Objective: to evaluate the effect of Ocular Bandage Gel (OBG) vs. Refresh ® Plus Preservative - Free Lubricant (RPF) in patients with punctate epitheliopathies (PE) due to dry eye. Day 0 Measure differences in outcomes Screening (n= 20 patients; 40 eyes) OBG (crosslinked HA eye drop) Standard - of - care (Refresh® Plus Preservative - Free Lubricant) Randomize Day 7 Fluorescein Stain 1 ° Endpoint Study Design • Two - arm, randomized, positive - controlled, investigator - masked • Seven - day run - in period followed by a fourteen - day treatment period • Patient acted as own control; one eye randomized to OBG arm and one eye randomized to RPF Outcome Measures Primary Endpoint: • Change in NEI corneal fluorescein staining score on Day 14 from baseline for central region and total region of the cornea Secondary Endpoints: • Blurriness: Change in tear film stability and visual acuity at 30 and 60 minutes post instilling drops Enrollment • 20 patients (40 eyes) where both eyes had to qualify for study randomization • Subset analysis completed on 16 patients (32 eyes) – applying additional criteria (central staining) Day - 7 Run - in period Day 14 Fluorescein Stain 1 ° Endpoint
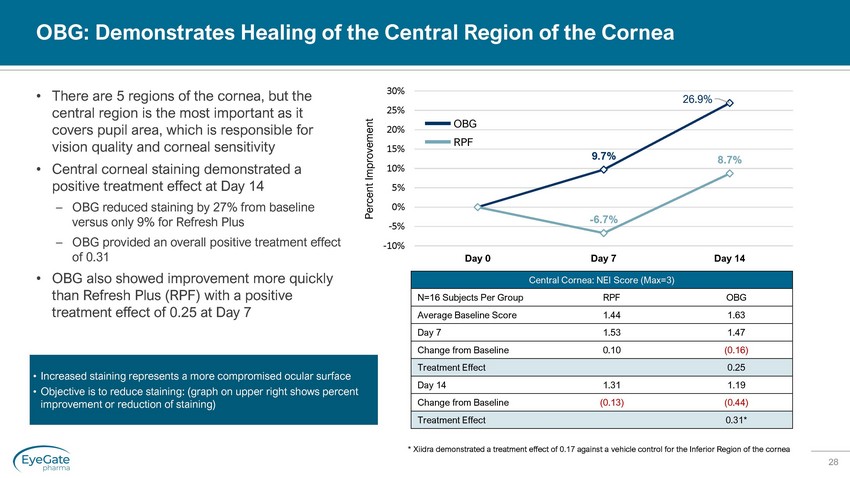
28 OBG: Demonstrates Healing of the Central Region of the Cornea • There are 5 regions of the cornea, but the central region is the most important as it covers pupil area, which is responsible for vision quality and corneal sensitivity • Central corneal staining demonstrated a positive treatment effect at Day 14 – OBG reduced staining by 27% from baseline versus only 9% for Refresh Plus – OBG provided an overall positive treatment effect of 0.31 • OBG also showed improvement more quickly than Refresh Plus (RPF) with a positive treatment effect of 0.25 at Day 7 Central Cornea: NEI Score (Max=3) N=16 Subjects Per Group RPF OBG Average Baseline Score 1.44 1.63 Day 7 1.53 1.47 Change from Baseline 0.10 (0.16) Treatment Effect 0.25 Day 14 1.31 1.19 Change from Baseline (0.13) (0.44) Treatment Effect 0.31* 9.7% 26.9% - 6.7% 8.7% -10% -5% 0% 5% 10% 15% 20% 25% 30% Day 0 Day 7 Day 14 Percent Improvement OBG RPF • Increased staining represents a more compromised ocular surface • Objective is to reduce staining: (graph on upper right shows percent improvement or reduction of staining) * Xiidra demonstrated a treatment effect of 0.17 against a vehicle control for the Inferior Region of the cornea
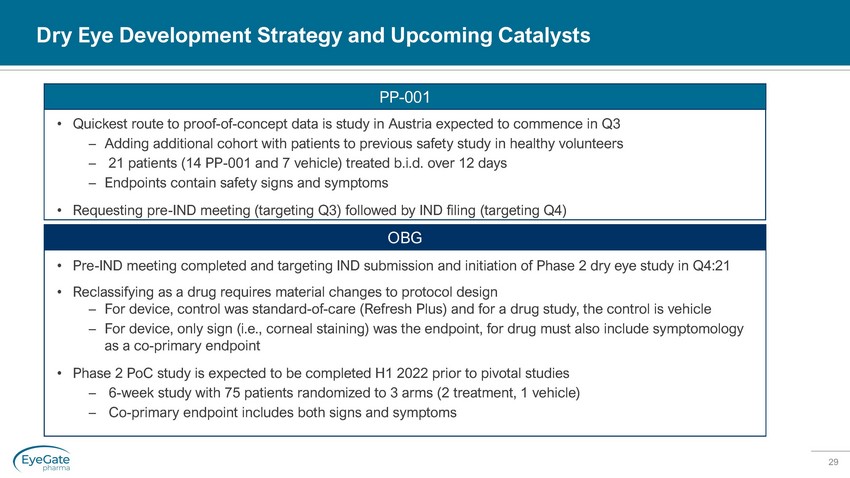
29 • Quickest route to proof - of - concept data is study in Austria expected to commence in Q3 – Adding additional cohort with patients to previous safety study in healthy volunteers – 21 patients (14 PP - 001 and 7 vehicle) treated b.i.d. over 12 days – Endpoints contain safety signs and symptoms • Requesting pre - IND meeting (targeting Q3) followed by IND filing (targeting Q4) PP - 001 • Pre - IND meeting completed and targeting IND submission and initiation of Phase 2 dry eye study in Q4:21 • Reclassifying as a drug requires material changes to protocol design – For device, control was standard - of - care (Refresh Plus) and for a drug study, the control is vehicle – For device, only sign (i.e., corneal staining) was the endpoint, for drug must also include symptomology as a co - primary endpoint • Phase 2 PoC study is expected to be completed H1 2022 prior to pivotal studies – 6 - week study with 75 patients randomized to 3 arms (2 treatment, 1 vehicle) – Co - primary endpoin t includes both signs and symptoms OBG Dry Eye Development Strategy and Upcoming Catalysts

30 ALLERGIC CONJUNCTIVITIS • Overview and PP - 001
31 • Conjunctiva is a thin, translucent membrane lining the anterior part of the sclera and inside of the eyelids • Conjunctivitis is characterized by inflammation and swelling of the conjunctival tissue, accompanied by engorgement of the blood vessels, ocular discharge, pain, and itchiness (i.e., allergies) • While there are many types of conjunctivitis, viral, allergic and bacterial are the three most common • Allergic Conjunctivitis – 66 million or more adults in the U.S. 1 – Mast cell stabilizers and/or antihistamines are first - line therapy 2 – Moderate conditions (i.e., symptoms and signs remain uncontrolled) require short - term steroids 2 – Severe conditions require immunotherapy to prevent tissue damage (no product approved) 2 PP - 001 being Developed for Allergic Conjunctivitis • Requesting pre - IND meeting (targeting Q4) followed by IND filing – Will initiate a Ph 2 PoC study for allergic conjunctivitis patients prior to year - end Development Strategy and Upcoming Catalysts 1. Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988 1994. J Allergy Clin Immunol . 2010;126(4):778 783 .e 6 2. Dupuis et al, A Contemporary look at Allergic Conjunctivitis, 2020, Allergy Asthma Clin Immunol 2020 16:5

32 OCULAR WOUND HEALING • Overview and OBG

33 Ocular Surface Wound Healing Market • Corneal epithelial and stromal defects are common ocular conditions (2 million annually in the U.S.) – Eye injuries represent up to 18% of emergency room traumas 1 – Corneal abrasions account for up to 4% of all U.S. occupational injuries 1 – Approximately 20% of patients with facial burns have ocular injury 1 • OBG accelerates the healing of the epithelium – OBG demonstrated acceleration of wound healing in patients that had undergone PRK surgery • Limited reimbursement for PRK indication • With OBG classified as a drug, will pivot development to Persistent Epithelial Defects (PED), a form of neurotrophic keratiti s – Only wound healing eye drop, Oxervate , recently approved for treatment of neurotrophic keratitis • Recombinant hNGF , applied 6X/day for 8 weeks (box arrives weekly, product packed in dry ice and requires preparation by patient) • Most common AE’s (>5%) are eye pain, ocular hyperemia, eye inflammation and increased lacrimation • Reimbursed at $90,000 per treatment regimen • Initiating a small open - label Proof - of - Concept study in H2 2021 in patients with Persistent Epithelial Defects (PED) Development Strategy and Upcoming Catalysts 1. Williams et al, Topical Cross - Linked HA - Based Hydrogel Accelerates Closure of Corneal Epithelial Defects and Repair of Stromal U lceration in Companion Animals iovs.arvojournals.org j ISSN: 1552 - 5783
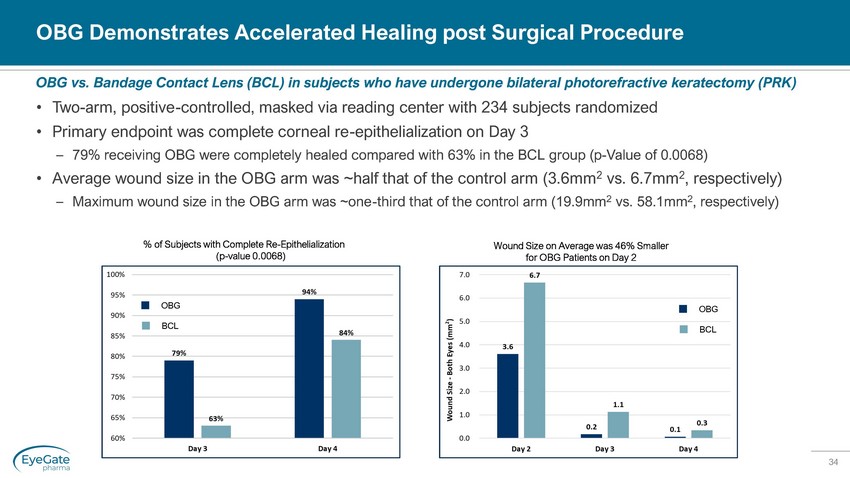
34 OBG Demonstrates Accelerated Healing post Surgical Procedure • Two - arm, positive - controlled, masked via reading center with 234 subjects randomized • Primary endpoint was complete corneal re - epithelialization on Day 3 – 79% receiving OBG were completely healed compared with 63% in the BCL group (p - Value of 0.0068) • Average wound size in the OBG arm was ~half that of the control arm (3.6mm 2 vs. 6.7mm 2 , respectively) – Maximum wound size in the OBG arm was ~one - third that of the control arm (19.9mm 2 vs. 58.1mm 2 , respectively) OBG vs. Bandage Contact Lens (BCL) in subjects who have undergone bilateral photorefractive keratectomy (PRK) % of Subjects with Complete Re - Epithelialization (p - value 0.0068) Wound Size on Average was 46% Smaller for OBG Patients on Day 2 OBG BCL OBG BCL
35 Anticipated Milestones • PP - 001 Ocular – Initiate POC dry eye study in Austria – Pre - IND meeting in U.S. for dry eye • PP - 001 Systemic – Topline P1 safety data (Austria) – IND filing in the U.S. and Initiate P1b/2a oncology study • PP - 001 Oral – File IND • PP - 001 Ocular – Topline P2 data • OBG – Topline P2 data in dry eye – Topline POC data in persistent epithelial defect patients • PP - 001 Ocular – Topline POC dry eye data – IND filing in U.S. for dry eye – Initiate P2 in U.S. for dry eye – IND filing in U.S. for allergic conjunctivitis • PP - 001 Systemic – Initiate P1 in Austria with IV formulation – Pre - IND meeting in U.S. for IV formulation • OBG – File IND for P2 study in dry eye – Initiate POC study in persistent epithelial defect patients Q3 2021 Q4 2021 1H 2022

36 May 2021