FWP: Filing under Securities Act Rules 163/433 of free writing prospectuses
Published on January 16, 2015

Eyegate Pharmaceuticals, Inc. Providing innovative products that enhance drug efficacy and patient compliance to improve vision Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration No. 333 - 197725 January 16, 2015

1 Forward Looking Statements Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s lead product EGP - 437, for the timing and outcome of the Company’s clinical trials, the potential approval to market EGP - 437, and the Company’s capital needs. Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation. Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful. The Company may consume more cash than it currently anticipates and faster than projected. Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates. If the FDA or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations. Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Registration Statement on Form S - 1, as amended to date. The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law.

2 Free Writing Prospectus Statement ▪ This presentation highlights basic information about us and the offering. Because it is a summary, it does not contain all of the information that you should consider before investing in our company. ▪ We have filed a registration statement (including a prospectus) with the United States Securities and Exchange Commission (SEC) for the offering to which this presentation relates. The registration statement has not yet become effective. Before you invest, you should read the prospectus in the registration statement (including the risk factors described therein) and other documents we have filed with the SEC for more complete information about us and the offering. You may get these documents, including the preliminary prospectus dated December 24, 2014, for free by visiting EDGAR on the SEC website at http://sec.gov. The preliminary prospectus, dated December 24, 2014, is available on the SEC website at http://www.sec.gov/Archives/edgar/data/1372514/000114420414075901/v395878_s1a.htm ▪ Alternatively, we or any underwriter participating in the offering will arrange to send you the prospectus if you contact Aegis Capital Corp., Prospectus Department, 810 Seventh Avenue, 18th Floor, New York, NY 10019, telephone 212 - 813 - 1010, email: prospectus@aegiscap.com
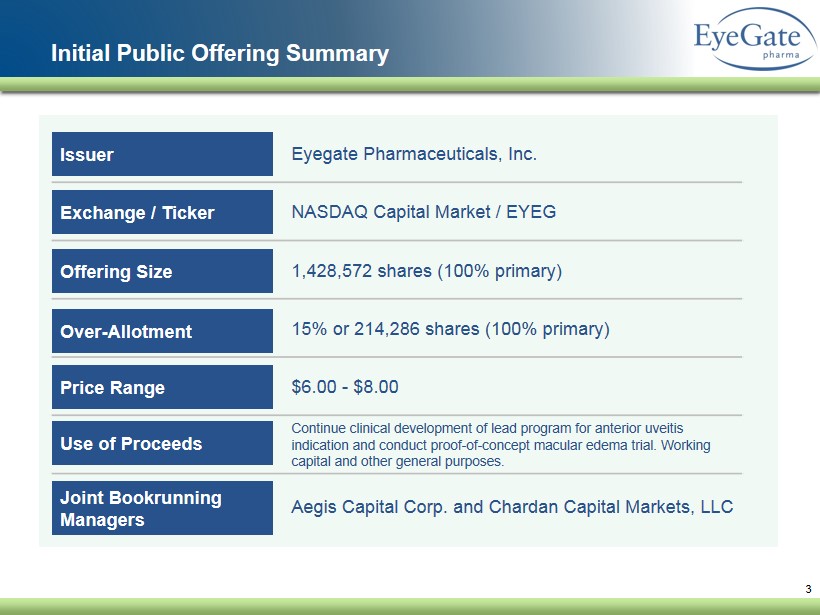
3 Initial Public Offering Summary Eyegate Pharmaceuticals, Inc. NASDAQ Capital Market / EYEG 1,428,572 shares (100% primary) 15% or 214,286 shares (100% primary) $6.00 - $8.00 Continue clinical development of lead program for anterior uveitis indication and conduct proof - of - concept macular edema trial. Working capital and other general purposes. Aegis Capital Corp. and Chardan Capital Markets, LLC Issuer Exchange / Ticker Offering Size Over - Allotment Price Range Use of Proceeds Joint Bookrunning Managers

4 Management Team ▪ Stephen From, President & CEO ▪ With EyeGate since October 2005 ▪ Former CFO, Centelion SAS (Aventis subsidiary) ▪ Healthcare investment Banker ▪ Qualified Chartered Accountant (PwC) ▪ Michael Manzo, VP Engineering ▪ With EyeGate since October 2006 ▪ Over 30 years of experience in product development and manufacturing in the medical device industry ▪ Former President and COO, Jenline Industries (now part of Helix Medical) ▪ Senior Marketing Engineer, ONUX Medical ▪ Lisa Brandano, Director, Clinical Operations ▪ Former Assistant Managing Director of the Boston office and Director, Clinical Trial Operations, CATO Research ▪ Beth Israel Deaconess Medical Center, Boston ▪ Michael Patane Ph.D., CSO (Consultant) ▪ Exec. Director, Global Discovery Chemistry, Novartis, responsible for infectious diseases and ophthalmology ▪ Director, Medicinal Discovery, Millennium Pharma ▪ Michael Raizman M.D., Consultant ▪ Specialist in Laser Vision Correction and Treatment of the Cornea, Ophthalmic Consultants of Boston ▪ Associate Professor of Ophthalmology, Tufts University

5 Selected Board Members ▪ Paul Chaney (Chairman) ▪ President and CEO of PanOptica , Inc. ▪ 25 years experience in pharmaceutical industry including several VP positions at Pharmacia ▪ Morton Goldberg M.D.* ▪ Joseph E. Green Professor of Ophthalmology at the Wilmer Eye Institute, Johns Hopkins University School of Medicine ▪ Former Director and William Holland Wilmer Professor of Ophthalmology, Wilmer Eye Institute ▪ Praveen Tyle PhD. ▪ President and CEO of Osmotica Pharma . ▪ SVP and Global Head Business Development & Licensing for Novartis Consumer Health OTC ▪ Former VP of R&D and Chief Scientific Officer for Bausch & Lomb Board of Directors with Ophthalmic Experience and Medical Advisors Selected Medical Advisors ▪ Victor Perez M.D . ▪ Director of the Ocular Surface Center and the Walter G. Ross Distinguished Chair in Ophthalmic Research Programs at the Bascom Palmer Eye Institute ▪ Professor of Ophthalmology, Microbiology and Immunology, University of Miami Miller School of Medicine ▪ John Sheppard M.D. ▪ President, Virginia Eye Consultants: Research , Education & Clinical Excellence ▪ Professor of Ophthalmology, Microbiology & Molecular Biology , Ophthalmology Residency Research Director, Clinical Director, Thomas R. Lee Center for Ocular Pharmacology, Eastern Virginia Medical School ▪ Stephen Foster M.D. ▪ Founder and Director of the Massachusetts Eye Research and Surgery Institute (MERSI) ▪ Author of over 500 published books and papers and has won numerous awards including The International Ocular Inflammation Society Award and The American Academy of Ophthalmology Senior Honor Award * Participation by Board Member does not constitute or imply endorsement by the Johns Hopkins University or the Johns Hopkins Ho spital and Health System

6 Company Overview ▪ Late - stage specialty pharmaceutical and drug delivery company targeting highly prevalent ocular indications ▪ Lead investors include Ventech , IPSA, Natixis ▪ Lead product candidate, EGP - 437 for anterior uveitis, matched response rate for standard of care in non - inferiority trial ▪ 505(b)(2) NDA filing scheduled for end of year 2016 ▪ EGP - 437 being evaluated for back - of - eye disease, like macular edema ▪ EyeGate II® Delivery System: Proprietary, non - invasive delivery platform; >1,700 treatments performed to - date ▪ System is approved through a 510(k) filing at time of drug NDA submission ▪ Easy to use; can be done by ophthalmologist or optometrist in <5 minutes ▪ Delivers small and large molecules to anterior or posterior of eye ▪ Significant patient and clinician advantages over drops or ocular injections ▪ Safer, lower - risk alternative to intravitreal injection
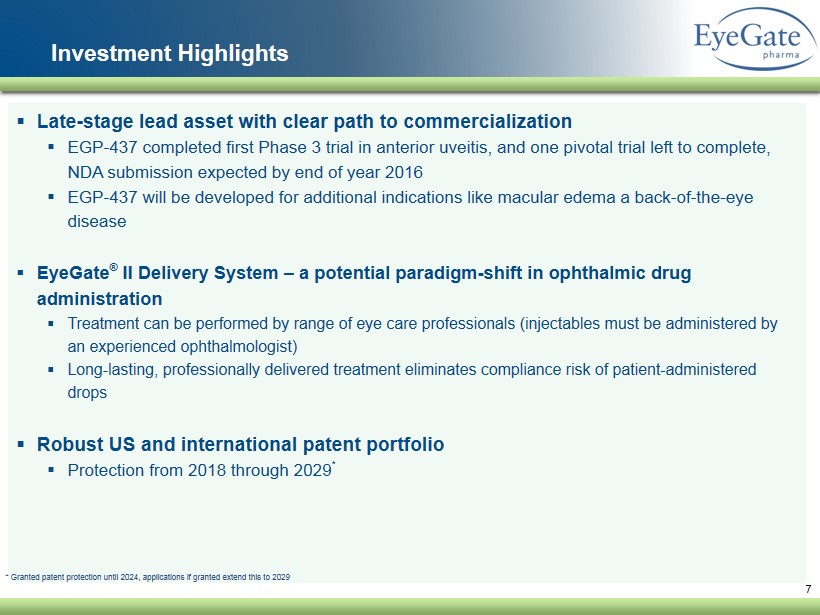
7 Investment Highlights ▪ Late - stage lead asset with clear path to commercialization ▪ EGP - 437 completed first Phase 3 trial in anterior uveitis, and one pivotal trial left to complete, NDA submission expected by end of year 2016 ▪ EGP - 437 will be developed for additional indications like macular edema a back - of - the - eye disease ▪ EyeGate ® II Delivery System – a potential paradigm - shift in ophthalmic drug administration ▪ Treatment can be performed by range of eye care professionals ( injectables must be administered by an experienced ophthalmologist) ▪ Long - lasting, professionally delivered treatment eliminates compliance risk of patient - administered drops ▪ Robust US and international patent portfolio ▪ Protection from 2018 through 2029 * * Granted patent protection until 2024, applications if granted extend this to 2029

8 Unique Ophthalmic Delivery Platform
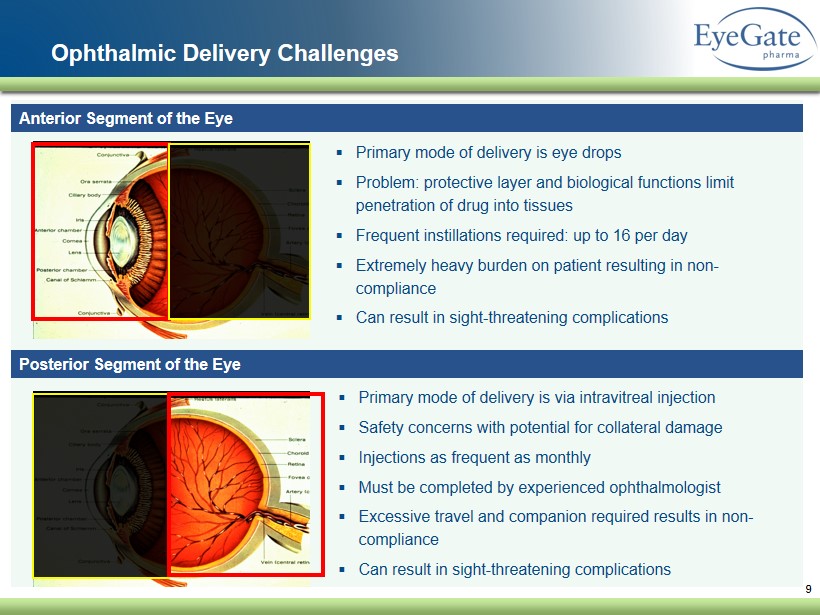
Ophthalmic Delivery Challenges Anterior Segment of the Eye Posterior Segment of the Eye ▪ Primary mode of delivery is eye drops ▪ Problem: protective layer and biological functions limit penetration of drug into tissues ▪ Frequent instillations required: up to 16 per day ▪ Extremely heavy burden on patient resulting in non - compliance ▪ Can result in sight - threatening complications ▪ Primary mode of delivery is via intravitreal injection ▪ Safety concerns with potential for collateral damage ▪ Injections as frequent as monthly ▪ Must be completed by experienced ophthalmologist ▪ Excessive travel and companion required results in non - compliance ▪ Can result in sight - threatening complications 9
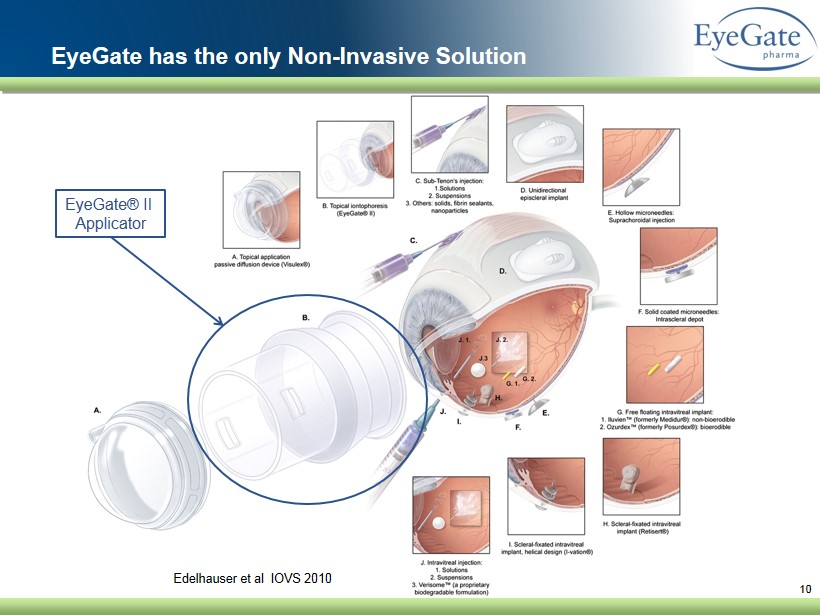
10 Edelhauser et al IOVS 2010 EyeGate has the only Non - Invasive Solution EyeGate® II Applicator
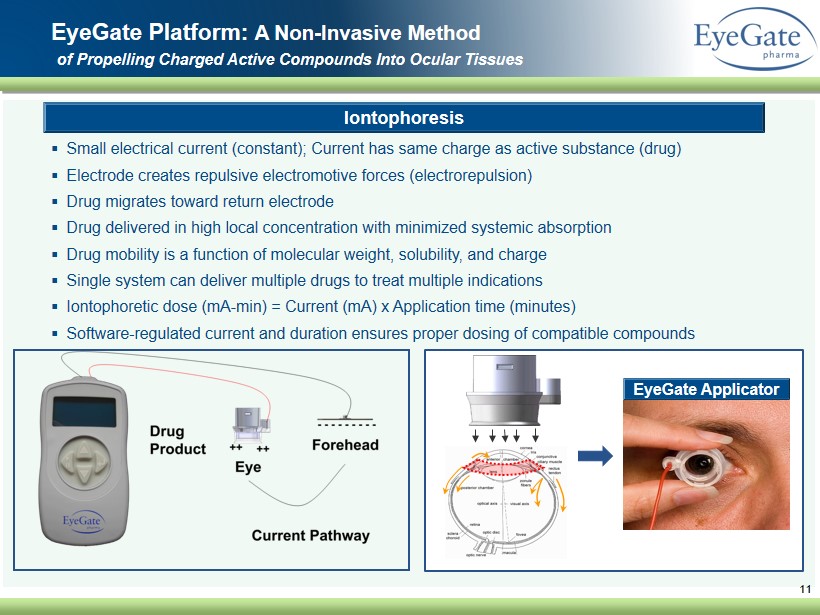
▪ Small electrical current (constant ); Current has same charge as active substance (drug) ▪ Electrode creates repulsive electromotive forces (electrorepulsion) ▪ Drug migrates toward return electrode ▪ Drug delivered in high local concentration with minimized systemic absorption ▪ Drug mobility is a function of molecular weight, solubility, and charge ▪ Single system can deliver multiple drugs to treat multiple indications ▪ Iontophoretic dose ( mA - min ) = Current (mA) x Application time (minutes ) ▪ Software - regulated current and duration ensures proper dosing of compatible compounds 11 EyeGate Platform: A Non - Invasive Method of Propelling Charged A ctive C ompounds I nto O cular Tissues Iontophoresis EyeGate Applicator
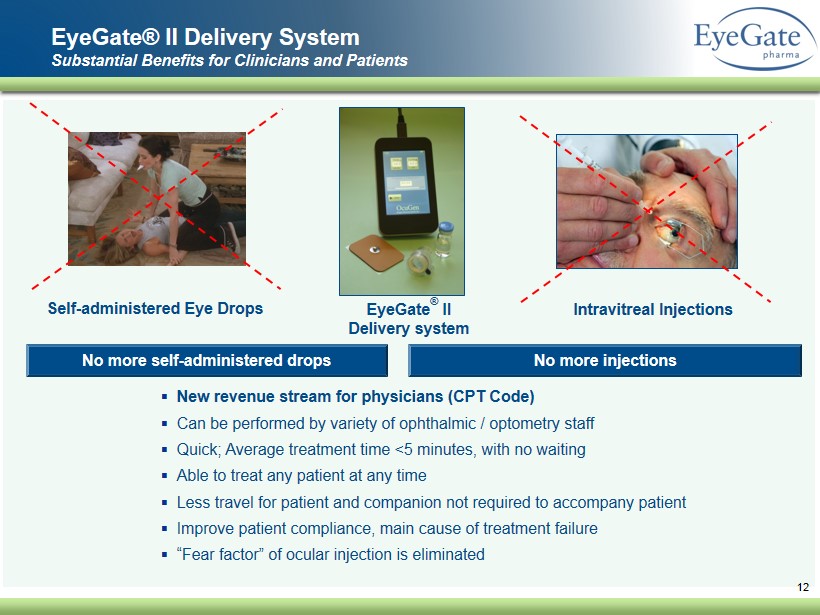
12 EyeGate ® II Delivery system No more self - administered drops ▪ New revenue stream for physicians (CPT Code) ▪ Can be performed by variety of ophthalmic / optometry staff ▪ Quick; Average treatment time <5 minutes, with no waiting ▪ Able to treat any patient at any time ▪ Less travel for patient and companion not required to accompany patient ▪ Improve patient compliance, main cause of treatment failure ▪ “ Fear factor” of ocular injection is eliminated No more injections Intravitreal Injections Self - administered Eye Drops EyeGate® II Delivery System Substantial Benefits for Clinicians and Patients
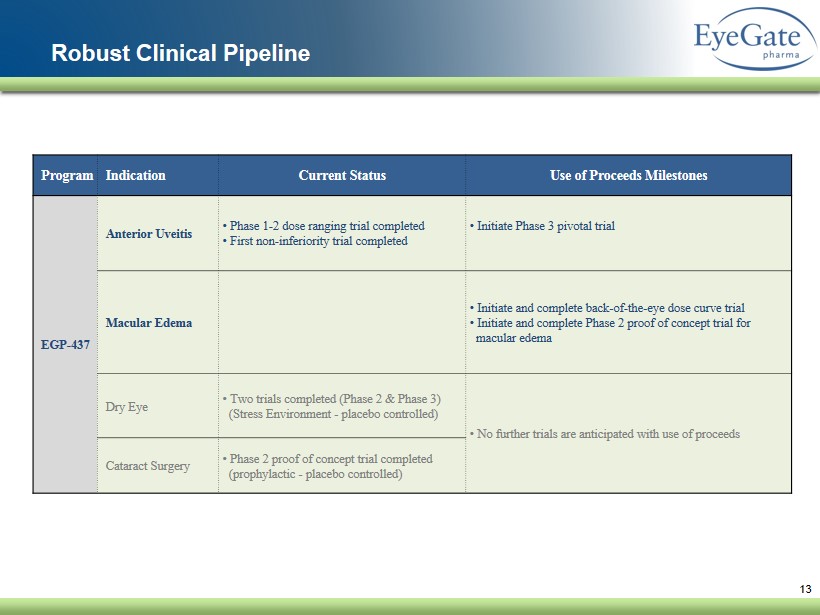
13 Robust Clinical Pipeline Program Indication Current Status Use of Proceeds Milestones EGP - 437 Anterior Uveitis • Phase 1 - 2 dose ranging trial completed • First non - inferiority trial completed • Initiate Phase 3 pivotal trial Macular Edema • Initiate and complete back - of - the - eye dose curve trial • Initiate and complete Phase 2 proof of concept trial for macular edema Dry Eye • Two trials completed (Phase 2 & Phase 3) (Stress Environment - placebo controlled) • No further trials are anticipated with use of proceeds Cataract Surgery • Phase 2 proof of concept trial completed (prophylactic - placebo controlled)

14 EGP - 437 Anti - Inflammatory
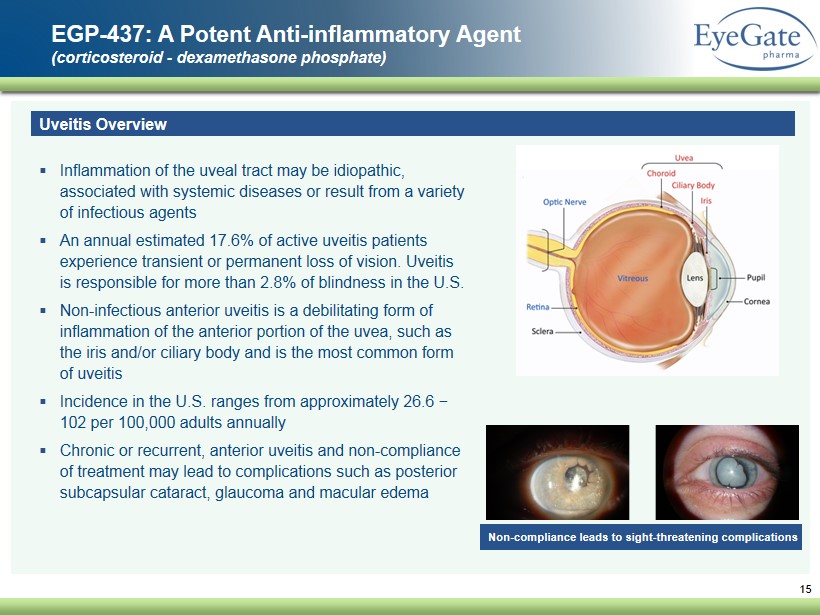
15 EGP - 437: A Potent A nti - inflammatory A gent (corticosteroid - dexamethasone phosphate) Uveitis Overview ▪ Inflammation of the uveal tract may be idiopathic, associated with systemic diseases or result from a variety of infectious agents ▪ An annual estimated 17.6% of active uveitis patients experience transient or permanent loss of vision. Uveitis is responsible for more than 2.8% of blindness in the U.S. ▪ Non - infectious anterior uveitis is a debilitating form of inflammation of the anterior portion of the uvea, such as the iris and/or ciliary body and is the most common form of uveitis ▪ Incidence in the U.S . ranges from approximately 26.6 − 102 per 100,000 adults annually ▪ Chronic or recurrent, anterior uveitis and non - compliance of treatment may lead to complications such as posterior subcapsular cataract, glaucoma and macular edema Non - compliance leads to sight - threatening complications
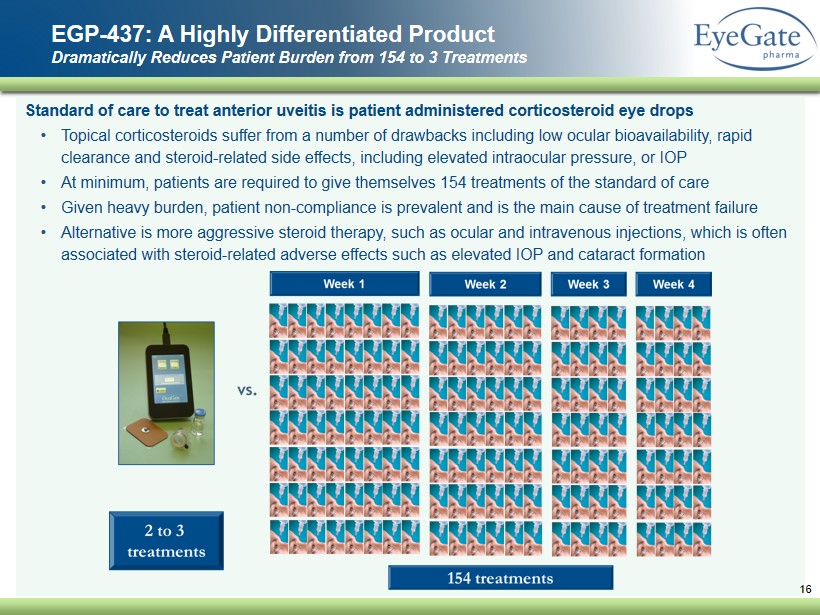
S tandard of care to treat anterior uveitis is patient administered corticosteroid eye drops • Topical corticosteroids suffer from a number of drawbacks including low ocular bioavailability, rapid clearance and steroid - related side effects, including elevated intraocular pressure, or IOP • At minimum , patients are required to give themselves 154 treatments of the standard of care • Given heavy burden , patient non - compliance is prevalent and is the main cause of treatment failure • Alternative is more aggressive steroid therapy, such as ocular and intravenous injections, which is often associated with steroid - related adverse effects such as elevated IOP and cataract formation EGP - 437: A Highly Differentiated Product Dramatically Reduces Patient Burden from 154 to 3 Treatments 16
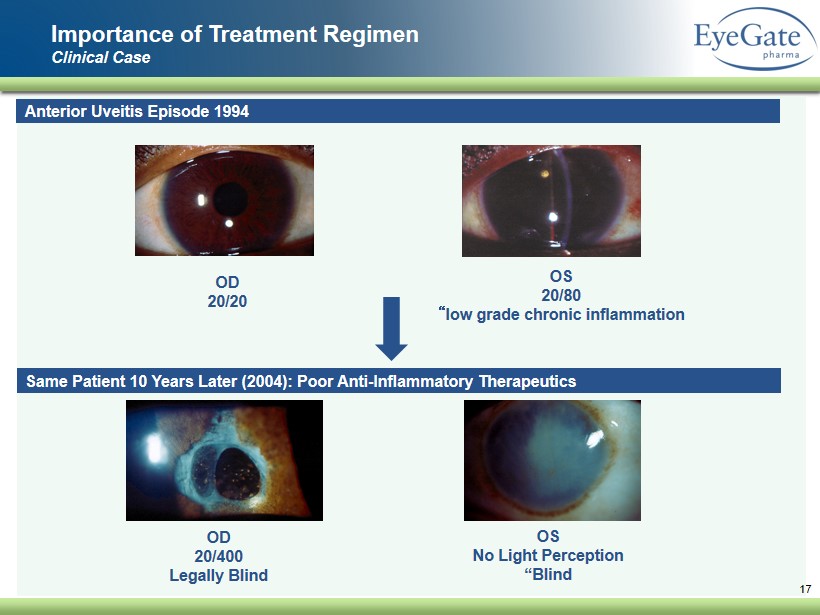
Importance of Treatment Regimen Clinical Case 17 Anterior Uveitis Episode 1994 OD 20/20 OS 20/80 “ low grade chronic inflammation Same Patient 10 Years Later (2004 ): Poor Anti - Inflammatory Therapeutics OD 20/ 400 Legally Blind OS No Light Perception “Blind
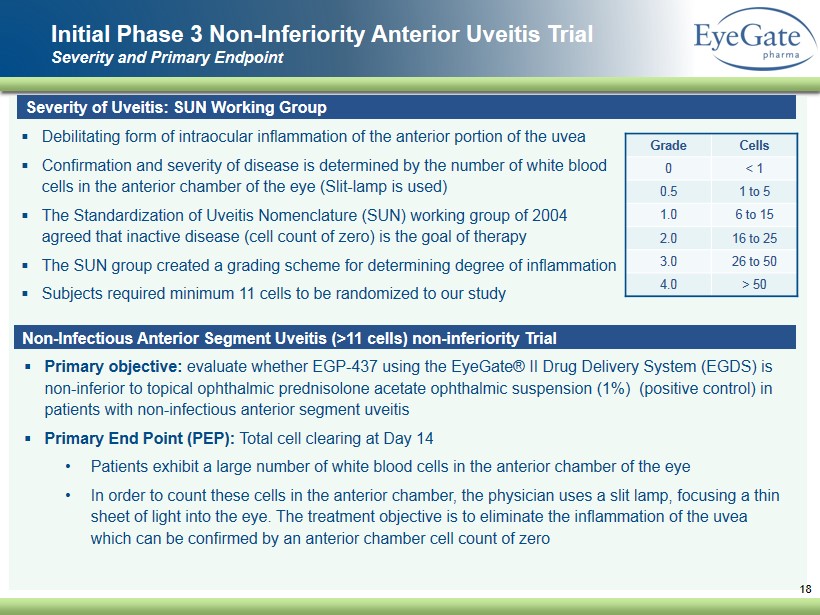
Initial Phase 3 Non - Inferiority A nterior U veitis Trial Severity and Primary Endpoint 18 Severity of Uveitis: SUN Working Group ▪ Debilitating form of intraocular inflammation of the anterior portion of the uvea ▪ Confirmation and severity of disease is determined by the number of white blood cells in the anterior chamber of the eye (Slit - lamp is used) ▪ The Standardization of Uveitis Nomenclature (SUN) working group of 2004 agreed that inactive disease (cell count of zero) is the goal of therapy ▪ The SUN group created a grading scheme for determining degree of inflammation ▪ Subjects required minimum 11 cells to be randomized to our study Grade Cells 0 < 1 0.5 1 to 5 1.0 6 to 15 2.0 16 to 25 3.0 26 to 50 4.0 > 50 Non - Infectious Anterior Segment Uveitis (>11 cells) non - inferiority Trial ▪ Primary objective: evaluate whether EGP - 437 using the EyeGate® II Drug Delivery System (EGDS) is non - inferior to topical ophthalmic prednisolone acetate ophthalmic suspension (1%) (positive control) in patients with non - infectious anterior segment uveitis ▪ Primary End Point (PEP): Total cell clearing at Day 14 • Patients exhibit a large number of white blood cells in the anterior chamber of the eye • In order to count these cells in the anterior chamber, the physician uses a slit lamp, focusing a thin sheet of light into the eye. The treatment objective is to eliminate the inflammation of the uvea which can be confirmed by an anterior chamber cell count of zero
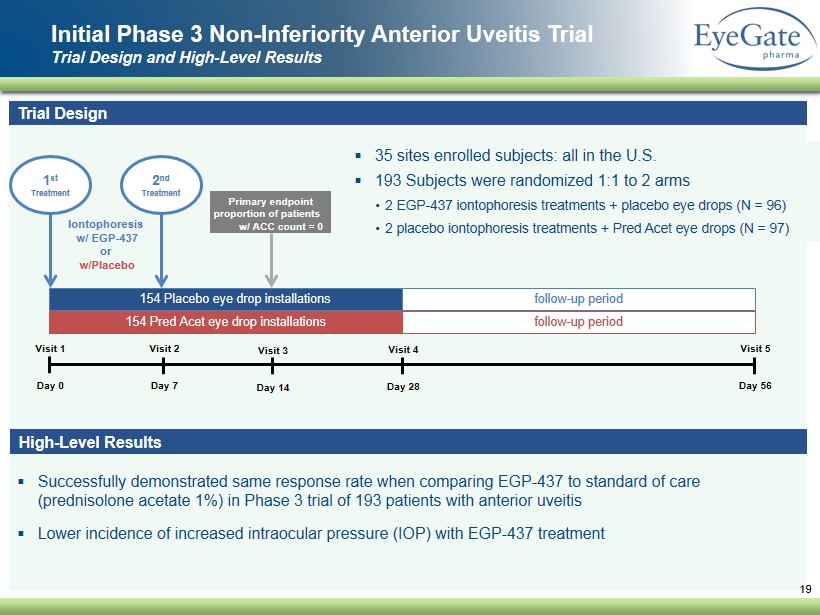
▪ Successfully demonstrated same response rate when comparing EGP - 437 to standard of care ( prednisolone acetate 1 %) in Phase 3 trial of 193 patients with anterior uveitis ▪ Lower incidence of increased intraocular pressure (IOP ) with EGP - 437 treatment Initial Phase 3 Non - Inferiority Anterior Uveitis Trial Trial Design and High - Level Results 19 Trial Design ▪ 35 sites enrolled subjects: all in the U.S. ▪ 193 Subjects were randomized 1:1 to 2 arms • 2 EGP - 437 iontophoresis treatments + placebo eye drops (N = 96) • 2 placebo iontophoresis treatments + Pred Acet eye drops (N = 97 ) Visit 1 Day 0 Visit 2 Day 7 Visit 3 Day 14 Visit 4 Day 28 Visit 5 Day 56 154 Pred Acet eye drop installations 154 Placebo eye drop installations vv follow - up period follow - up period 1 st Treatment Iontophoresis w/ EGP - 437 or w/Placebo Primary endpoint proportion of patients w/ ACC count = 0 2 nd Treatment High - Level Results
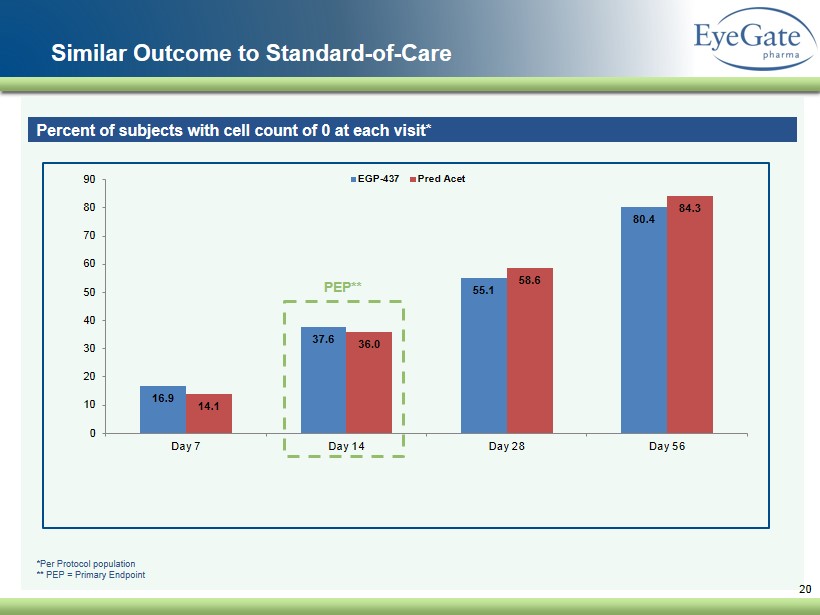
Similar Outcome to Standard - of - Care 20 Percent of subjects with cell count of 0 at each visit* 16.9 37.6 55.1 80.4 14.1 36.0 58.6 84.3 0 10 20 30 40 50 60 70 80 90 Day 7 Day 14 Day 28 Day 56 EGP-437 Pred Acet PEP** *Per Protocol population ** PEP = Primary Endpoint
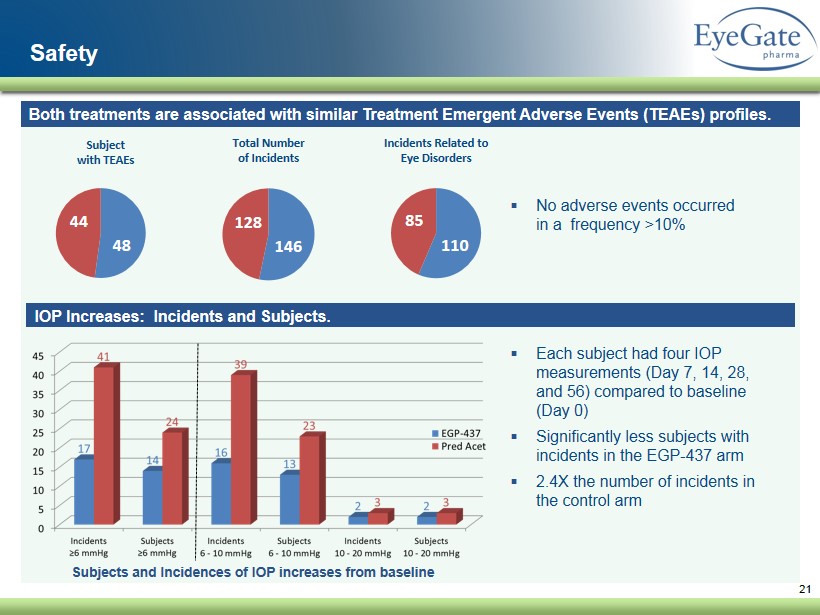
21 Safety ▪ Each subject had four IOP measurements (Day 7, 14, 28, and 56) compared to baseline (Day 0 ) ▪ Significantly less subjects with incidents in the EGP - 437 arm ▪ 2.4X the number of incidents in the control arm 146 128 Total Number of Incidents 110 85 Incidents Related to Eye Disorders Both treatments are associated with similar Treatment Emergent Adverse Events ( TEAEs) profiles . ▪ No adverse events occurred in a frequency >10% IOP Increases: Incidents and Subjects. Subjects and Incidences of IOP increases from baseline 48 44 Subject with TEAEs
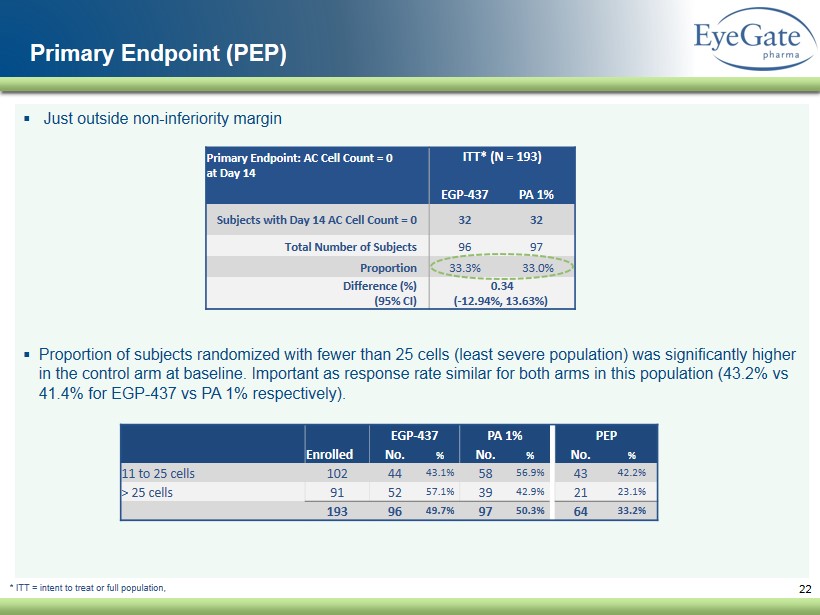
▪ Just outside non - inferiority margin ▪ Proportion of subjects randomized with fewer than 25 cells (least severe population) was significantly higher in the control arm at baseline. Important as response rate similar for both arms in this population (43.2% vs 41.4% for EGP - 437 vs PA 1% respectively). 22 Primary Endpoint (PEP) Primary Endpoint: AC Cell Count = 0 at Day 14 ITT* (N = 193) EGP - 437 PA 1% Subjects with Day 14 AC Cell Count = 0 32 32 Total Number of Subjects 96 97 Proportion 33.3% 33.0 % Difference (%) (95% CI) 0.34 ( - 12.94%, 13.63%) * ITT = intent to treat or full population, EGP - 437 PA 1% PEP Enrolled No. % No. % No. % 11 to 25 cells 102 44 43.1% 58 56.9% 43 42.2% > 25 cells 91 52 57.1% 39 42.9% 21 23.1% 193 96 49.7% 97 50.3% 64 33.2%
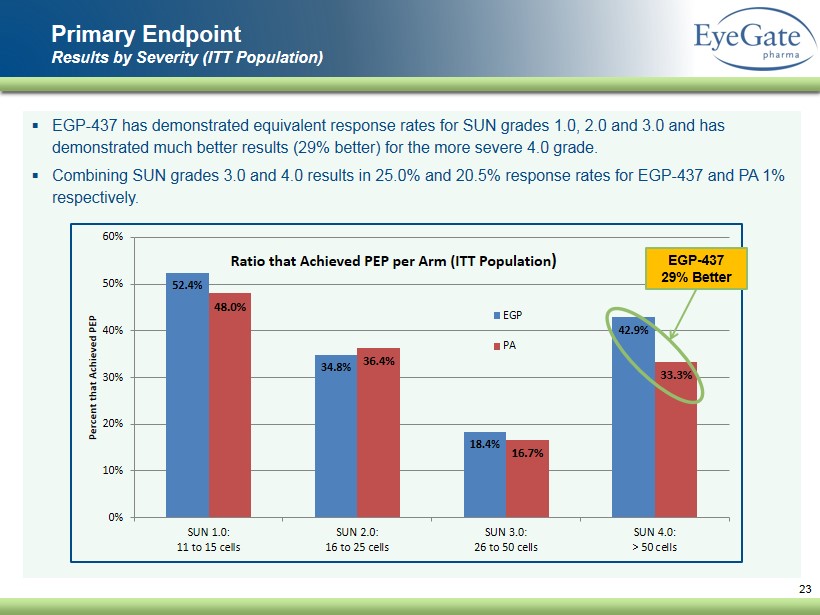
▪ EGP - 437 has demonstrated equivalent response rates for SUN grades 1.0, 2.0 and 3.0 and has demonstrated much better results (29% better) for the more severe 4.0 grade. ▪ Combining SUN grades 3.0 and 4.0 results in 25.0% and 20.5% response rates for EGP - 437 and PA 1% respectively. Primary Endpoint Results by Severity (ITT Population) 23 52.4% 34.8% 18.4% 42.9% 48.0% 36.4% 16.7% 33.3% 0% 10% 20% 30% 40% 50% 60% SUN 1.0: 11 to 15 cells SUN 2.0: 16 to 25 cells SUN 3.0: 26 to 50 cells SUN 4.0: > 50 cells Percent that Achieved PEP Ratio that Achieved PEP per Arm (ITT Population ) EGP PA EGP - 437 29% Better
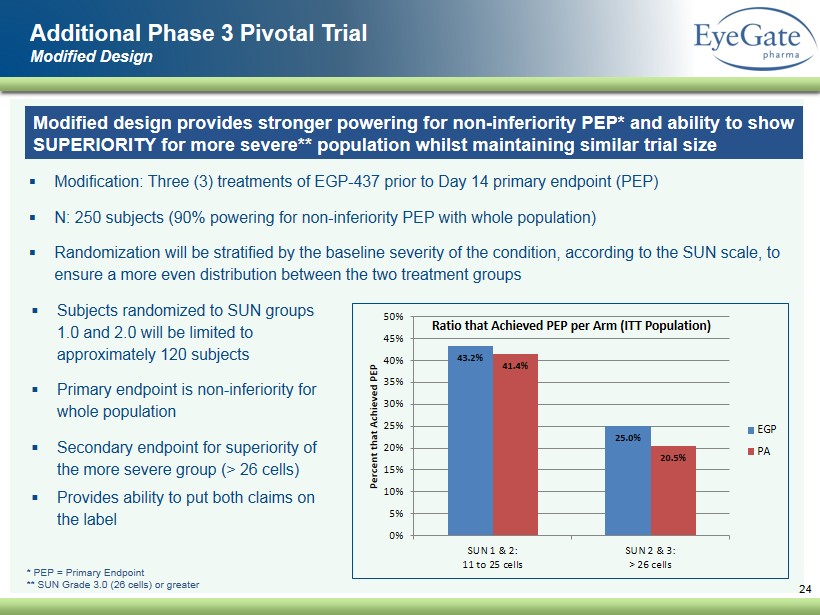
▪ Modification: Three (3) treatments of EGP - 437 prior to Day 14 primary endpoint (PEP) ▪ N: 250 subjects (90% powering for non - inferiority PEP with whole population) ▪ Randomization will be stratified by the baseline severity of the condition, according to the SUN scale, to ensure a more even distribution between the two treatment groups 24 Additional Phase 3 Pivotal Trial Modified Design * PEP = Primary Endpoint ** SUN Grade 3.0 (26 cells) or greater 43.2% 25.0% 41.4% 20.5% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% SUN 1 & 2: 11 to 25 cells SUN 2 & 3: > 26 cells Percent that Achieved PEP Ratio that Achieved PEP per Arm (ITT Population) EGP PA ▪ Subjects randomized to SUN groups 1.0 and 2.0 will be limited to approximately 120 subjects ▪ Primary endpoint is non - inferiority for whole population ▪ Secondary endpoint for superiority of the more severe group (> 26 cells) ▪ Provides ability to put both claims on the label Modified design provides stronger powering for non - inferiority PEP* and ability to show SUPERIORITY for more severe ** population whilst maintaining similar trial size
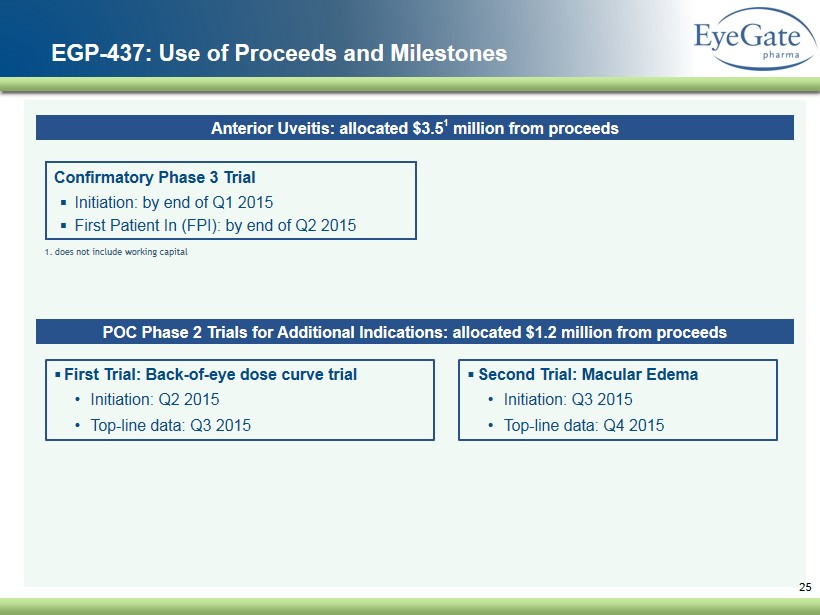
25 EGP - 437: Use of Proceeds and Milestones POC Phase 2 Trials for Additional Indications: allocated $1.2 million from proceeds Anterior Uveitis: allocated $3.5 1 million from proceeds Confirmatory Phase 3 Trial ▪ Initiation: by end of Q1 2015 ▪ First Patient In (FPI): by end of Q2 2015 1. does not include working capital ▪ First Trial: Back - of - eye dose curve trial • Initiation: Q2 2015 • Top - line data: Q3 2015 ▪ Second Trial: Macular Edema • Initiation: Q3 2015 • Top - line data: Q4 2015

26 ▪ Clearly - defined and sufficiently concentrated universe of clinicians that treat anterior uveitis in the U . S . which can be addressed by small, focused specialty sales team ▪ Plan to build key capabilities to support specialty sales team ▪ Marketing, market access, sales management and medical affairs ▪ Opportunity to grow overall market by enabling new point - of - care options ▪ Injectable therapies limit administration to experienced ophthalmologist only ▪ EGP - 437 via EyeGate® II Delivery System can be administered by optometrist, nurse and other staff ▪ Incremental opportunity through international commercialization ▪ Intend to enter into strategic collaboration for commercialization outside the U . S . EGP - 437 Commercial Strategy

27 ▪ Includes device and drug ▪ Combining drug vial and device disposables together: • Ensures use of approved drug with applicator • Provides simplification of inventory and invoicing ▪ Shelf - life established at 24 months (drug and applicator) ▪ CPT Code: In addition to office reimbursement, clinic receives reimbursement for performing treatment ▪ J - code: The kit (drug + disposables) will be billed under a J - code Payment that would be based on ASP ( price we establish) + x% for the kit. EyeGate sells kit to clinic Reimbursement Reimbursement: In - office treatment and physician office billing involves multiple code sets. Single - Use Kit
28 Protection from 2018 through 2029* Strong Patent P ortfolio Ten families (72 patents granted) ▪ Eight belong to the “ever growing” delivery system patent portfolio • 13 U.S. and 58 foreign patents granted • 3 U.S. and 16 foreign pending applications ▪ Two relate to drug compositions and treatments utilizing delivery system: • 1 foreign patent granted • 3 U.S. and 6 foreign applications * Granted patent protection until 2024, applications if granted extend this to 2029

29 ▪ Foam reservoir for drug ▪ Circular design ▪ Therapeutic supplied in separate vial ▪ Transfer system required to fill applicator EyeGate ® II Next Generation ▪ Pre - filled with therapeutic ▪ Eliminates need for transfer system ▪ Smaller package design ▪ Oval ocular interface facilitates placement Current Eyegate ® II profile Evolution of a Platform: Applicator New IP generated for device and therapeutic – extending market exclusivity period
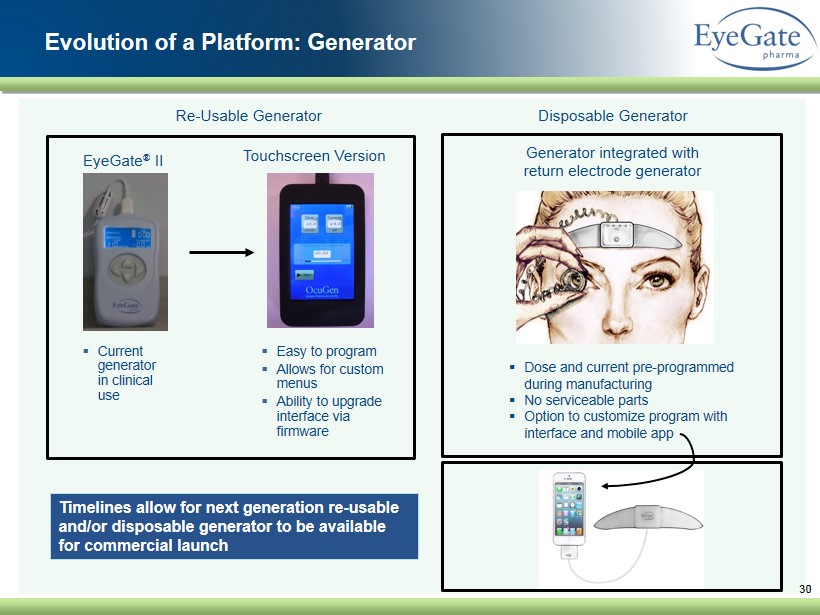
30 ▪ Current generator in clinical use EyeGate ® II ▪ Easy to program ▪ Allows for custom menus ▪ Ability to upgrade interface via firmware Timelines allow for next generation re - usable and/or disposable generator to be available for commercial launch Re - Usable Generator Disposable Generator ▪ Dose and current pre - programmed during manufacturing ▪ No serviceable parts ▪ Option to customize program with interface and mobile app Touchscreen Version Generator integrated with return electrode generator Evolution of a Platform: Generator
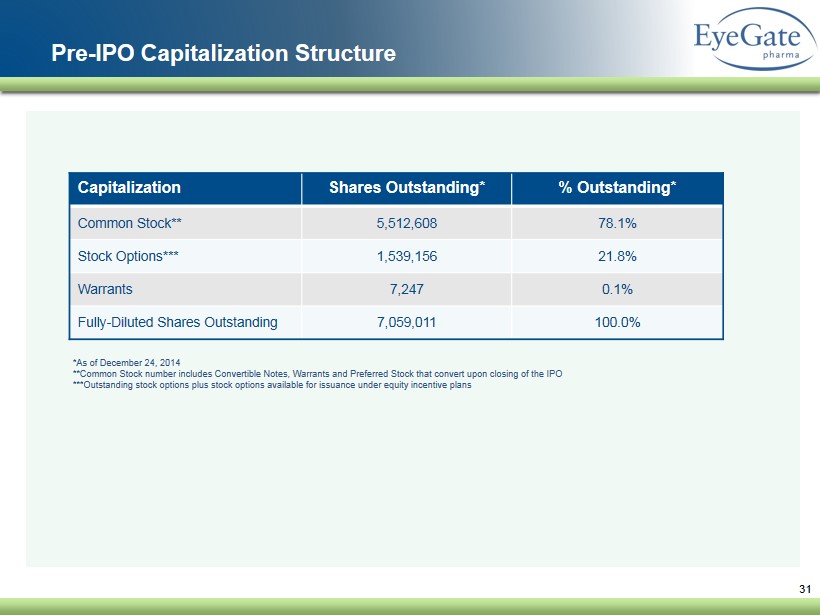
31 Pre - IPO Capitalization Structure Capitalization Shares Outstanding % Outstanding Common Stock* 5,512,608 78.1% Stock Options** 1,539,156 21.8% Warrants 7,247 0.1% Fully - Diluted Shares Outstanding 7,059,011 100.0% *Common Stock number includes Convertible Notes, Warrants and Preferred Stock that convert upon closing of the IPO **Outstanding stock options plus stock options available for issuance under equity incentive plans
32 Investment Highlights ▪ Late - stage lead asset with clear path to commercialization ▪ EGP - 437 completed first Phase 3 trial in anterior uveitis, and one pivotal trial left to complete, NDA submission expected by end of year 2016 ▪ EGP - 437 will be developed for additional indications like macular edema a back - of - the - eye disease ▪ EyeGate ® II Delivery System – a potential paradigm - shift in ophthalmic drug administration ▪ Treatment can be performed by range of eye care professionals ( injectables must be administered by an experienced ophthalmologist) ▪ Long - lasting, professionally delivered treatment eliminates compliance risk of patient - administered drops ▪ Robust US and international patent portfolio ▪ Protection from 2018 through 2029 * * Granted patent protection until 2024, applications if granted extend this to 2029