FWP: Filing under Securities Act Rules 163/433 of free writing prospectuses
Published on July 14, 2022

Kiora Pharmaceuticals, Inc. NASDAQ: KPRX Q3 2022 Filed pursuant to Rule 433 under the Securities Act of 1933, as amended Dated 7/14/2022 Registration Statement No. 333 - 264641

2 Forward Looking Statements Some of the statements in this presentation are “forward - looking” and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995 . These “forward - looking” statements include statements relating to, among other things, the development and commercialization efforts and other regulatory or marketing approval efforts pertaining to Kiora’s products, including KIO - 101 , KIO - 201 and KIO - 301 , as well as the success thereof, with such approvals or success may not be obtained or achieved on a timely basis or at all . These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this presentation, including, among other things, market and other conditions and certain risk factors described under the heading “Risk Factors” contained in Kiora’s Amended Annual Report on Form 10 - K/A filed with the SEC on July 7 , 2022 or described in Kiora’s other public filings . Kiora’s results may also be affected by factors of which Kiora is not currently aware . The forward - looking statements in this presentation speak only as of the date of this presentation . Kiora expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions, or circumstances on which any such statement is based, except as required by law .

3 Risk Factors

4 Free Writing Prospectus This presentation highlights basic information about the Company and the offering . Because it is a summary that has been prepared solely for informational purposes, it does not contain all of the information that you should consider before investing in our Company . Except as otherwise indicated, this presentation speaks only as of the date hereof . This presentation does not constitute an offer to sell, nor a solicitation of an offer to buy, any securities by any person in any jurisdiction in which it is unlawful for such person to make such an offering or solicitation . Neither the United States Securities and Exchange Commission (the “SEC”) nor any other regulatory body has approved or disapproved of our securities or passed upon the accuracy or adequacy of this presentation . Any representation to the contrary is a criminal offense . This presentation includes industry and market data that we obtained from industry publications and journals, third - party studies and surveys, internal company studies and surveys, and other publicly available information . Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable . Although we believe the industry and market data to be reliable as of the date of this presentation, this information could prove to be inaccurate . Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties . In addition, we do not know all of the assumptions that were used in preparing the forecasts from the sources relied upon or cited herein . We have filed a registration statement on Form S - 1 (File No . 333 - 264641 ) with the SEC, including a preliminary prospectus dated July 13 , 2022 (the “Preliminary Prospectus”), with respect to the offering of our securities to which this communication relates . Before you invest, you should read the Preliminary Prospectus (including the risk factors described therein) in the registration statement and, when available, the final prospectus relating to the offering, and the other documents we have filed with the SEC, for more complete information about the Company and the offering . You may obtain these documents, including the Preliminary Prospectus, for free by visiting EDGAR on the SEC website at http : //www . sec . gov . Alternatively, copies of the prospectus may be obtained, when available, from : Ladenburg Thalmann & Co . Inc . by written request addressed to Syndicate Department, 640 5 th Avenue, 4 th Floor, New York, NY 10019 (telephone number 1 - 800 - 573 - 2541 ) or by emailing prospectus@ladenburg . com .
5 Development stage company focused on rare & underserved ophthalmic diseases New executive leadership KIO - 301: Small molecule to restore vision in Retinitis Pigmentosa (RP) KIO - 101: Small molecule to treat Ocular Presentation of Rheumatoid Arthritis (OPRA) bm] Opportunity for value through multiple assets under development Corporate Overview KIO - 101, KIO - 201 & KIO - 301 are currently in clinical development and not commercially available
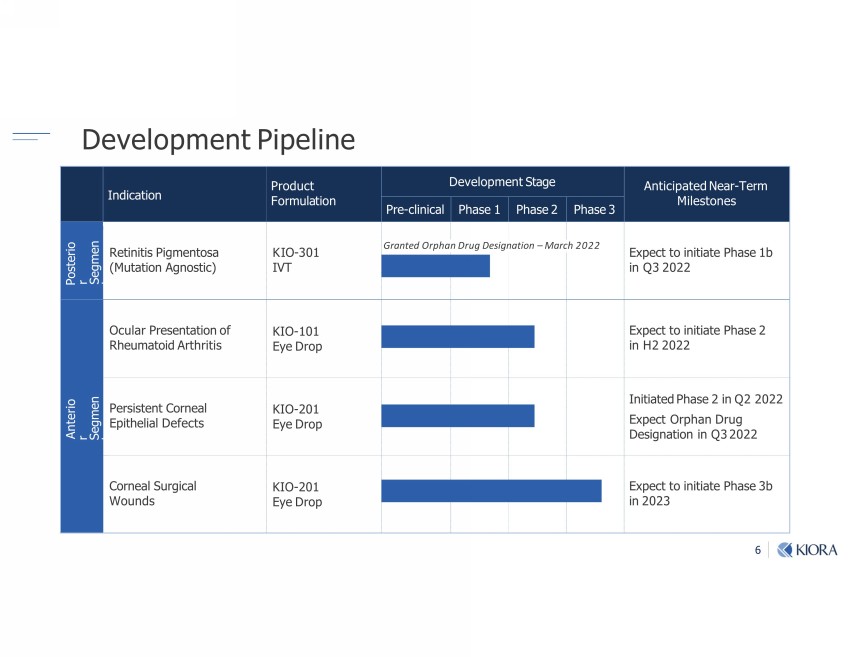
6 Indication Product Formulation Development Stage Anticipated Near - Term Milestones Pre - clinical Phase 1 Phase 2 Phase 3 Posterior Segment Retinitis Pigmentosa (Mutation Agnostic) KIO - 301 IVT Expect to initiate Phase 1b in Q3 2022 Anterior Segment Ocular Presentation of Rheumatoid Arthritis KIO - 101 Eye Drop Expect to initiate Phase 2 in H2 2022 Persistent Corneal Epithelial Defects KIO - 201 Eye Drop Initiated Phase 2 in Q2 2022 Expect Orphan Drug Designation in Q3 2022 Corneal Surgical Wounds KIO - 201 Eye Drop Expect to initiate Phase 3b in 2023 Development Pipeline
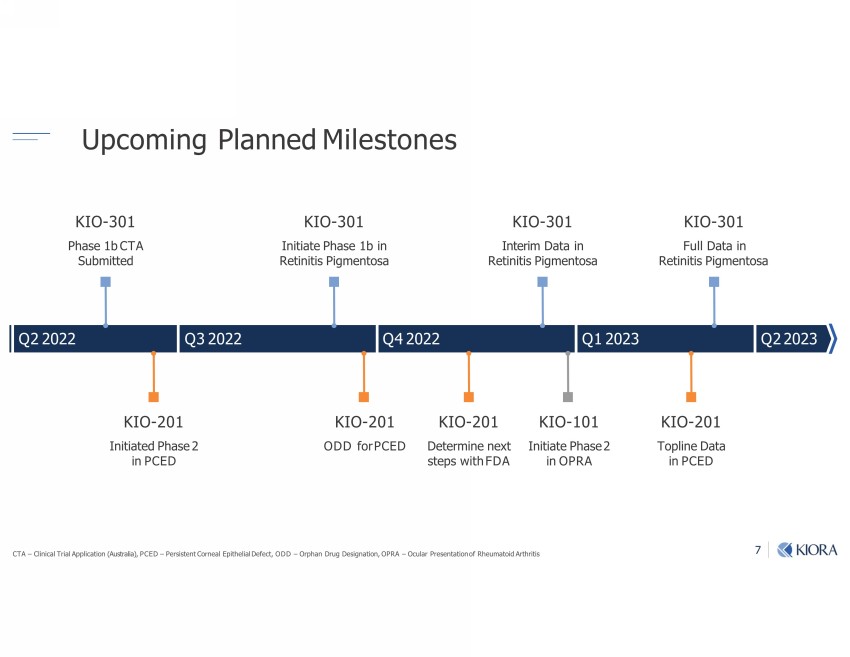
7 Upcoming Planned Milestones CTA – Clinical Trial Application (Australia), PCED – Persistent Corneal Epithelial Defect, ODD – Orphan Drug Designation, OPRA – Ocular Presentation of Rheumatoid Arthritis Q2 2022 Q3 2022 Q4 2022 Q1 2023 KIO - 301 Phase 1b CTA Submitted KIO - 301 Full Data in Retinitis Pigmentosa KIO - 301 Interim Data in Retinitis Pigmentosa KIO - 301 Initiate Phase 1b in Retinitis Pigmentosa KIO - 201 Initiated Phase 2 in PCED KIO - 201 Topline Data in PCED KIO - 201 Determine next steps with FDA KIO - 101 Initiate Phase 2 in OPRA

8 KIO - 301 Target Population: Retinitis Pigmentosa Product: Small Molecule Photoswitch for Retinal Reanimation IP: Anticipated through 2041* * Also granted 7 years orphan drug regulatory exclusivity
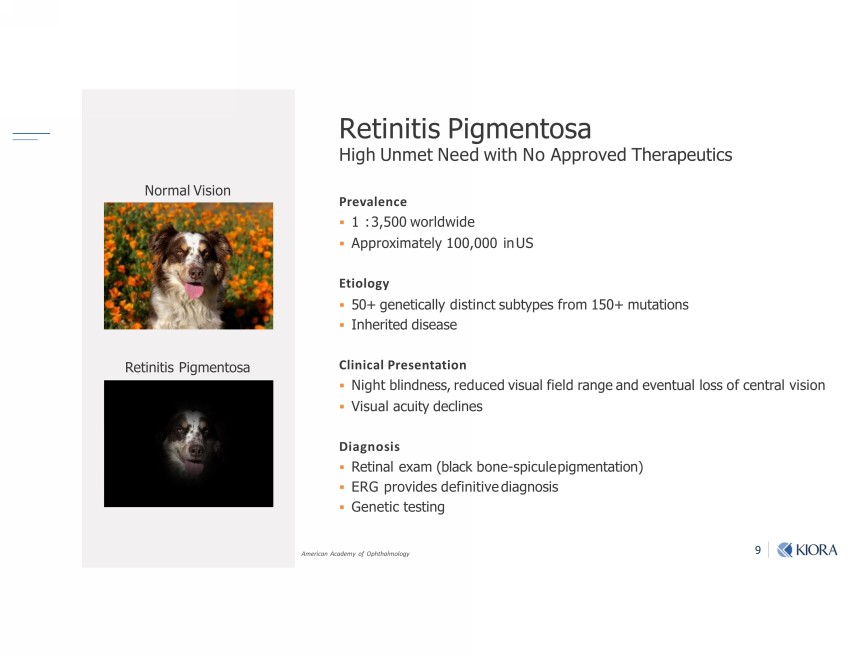
9 Prevalence ▪ 1 : 3,500 worldwide ▪ Approximately 100,000 in US Etiology ▪ 50+ genetically distinct subtypes from 150+ mutations ▪ Inherited disease Clinical Presentation ▪ Night blindness, reduced visual field range and eventual loss of central vision ▪ Visual acuity declines Diagnosis ▪ Retinal exam (black bone - spicule pigmentation) ▪ ERG provides definitive diagnosis ▪ Genetic testing Retinitis Pigmentosa High Unmet Need with No Approved Therapeutics Normal Vision Retinitis Pigmentosa
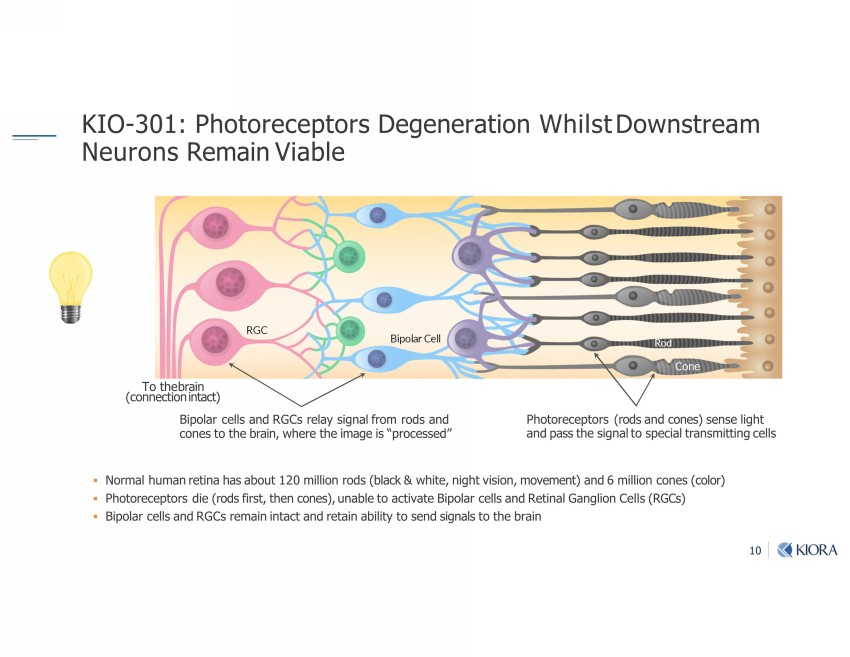
10 KIO - 301: Photoreceptors Degeneration Whilst Downstream Neurons Remain Viable ▪ Normal human retina has about 120 million rods (black & white, night vision, movement) and 6 million cones (color) ▪ Photoreceptors die (rods first, then cones), unable to activate Bipolar cells and Retinal Ganglion Cells (RGCs) ▪ Bipolar cells and RGCs remain intact and retain ability to send signals to the brain Photoreceptors (rods and cones) sense light and pass the signal to special transmitting cells Bipolar cells and RGCs relay signal from rods and cones to the brain, where the image is “processed” Bipolar Cell To the brain (connection intact) Cone Rod Rod Cone
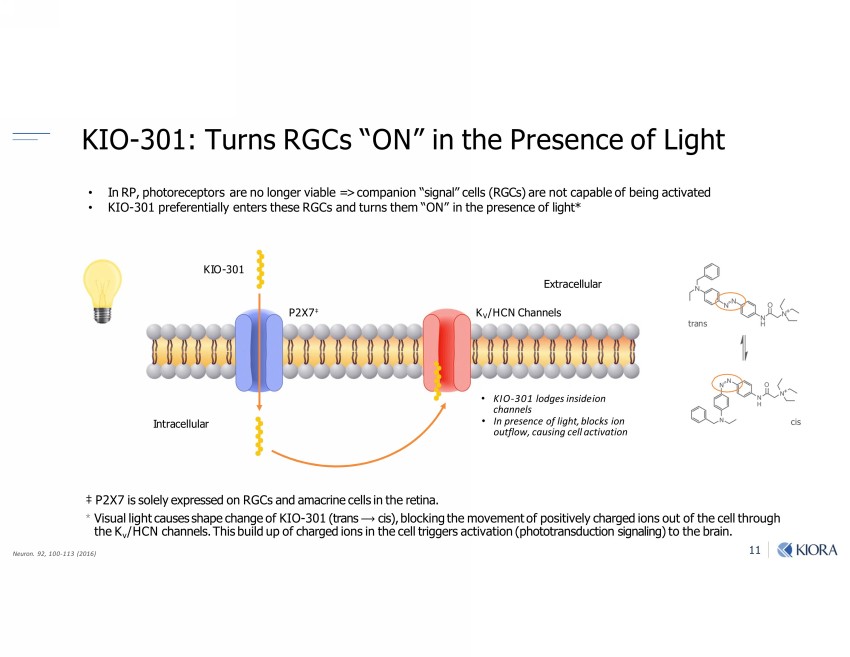
11 trans cis • In RP, photoreceptors are no longer viable => companion “signal” cells (RGCs) are not capable of being activated • KIO - 301 preferentially enters these RGCs and turns them “ON” in the presence of light* ‡ P2X7 is solely expressed on RGCs and amacrine cells in the retina. * Visual light causes shape change of KIO - 301 (trans ⟶ cis), blocking the movement of positively charged ions out of the cell through the K v /HCN channels. This build up of charged ions in the cell triggers activation (phototransduction signaling) to the brain. Intracellular KIO - 301 P2X7 ‡ -u K V /HCN Channels • KIO - 301 lodges inside ion channels KIO - 301: Turns RGCs “ON” in the Presence of Light • In presence of light, blocks ion outflow, causing cell activation
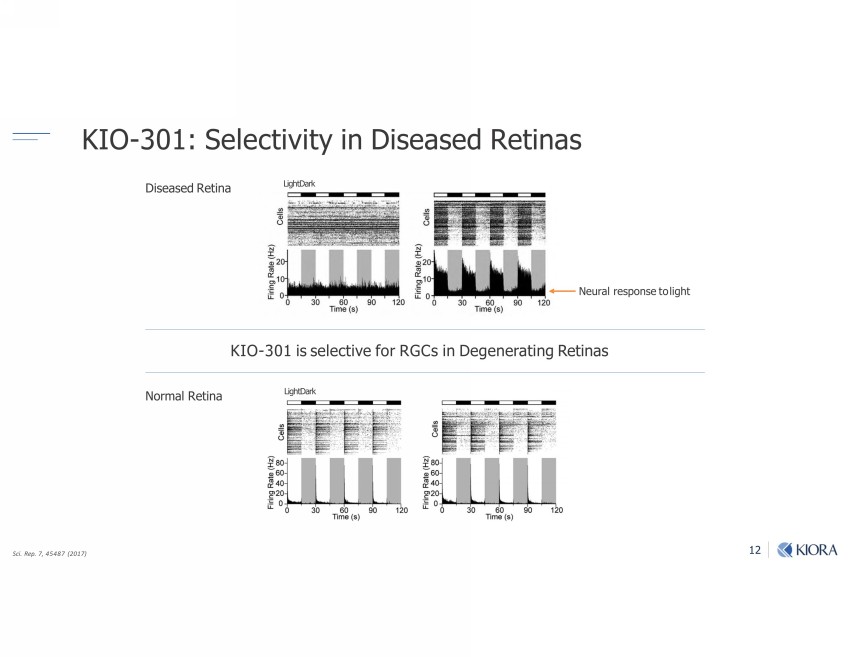
12 KIO - 301: Selectivity in Diseased Retinas Sci. Rep. 7, 45487 (2017) KIO - 301 is selective for RGCs in Degenerating Retinas Normal Retina Diseased Retina Light Dark Light Dark
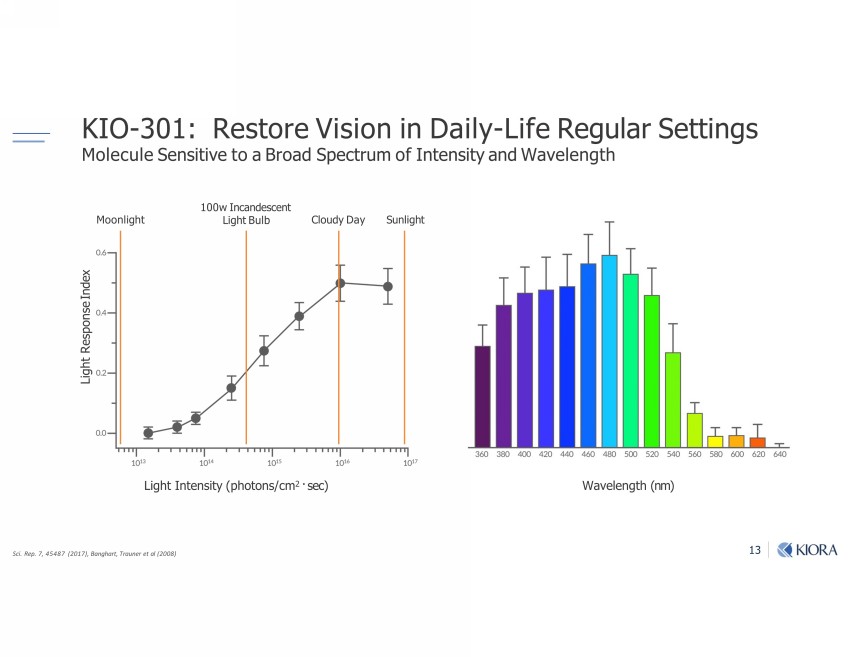
13 Moonlight ƐƏƏ Cloudy Day Sunlight Light Intensity (photons/cm 2 · sec) KIO - 301: Restore Vision in Daily - Life Regular Settings Molecule Sensitive to a Broad Spectrum of Intensity and Wavelength Sci. Rep. 7, 45487 (2017), Banghart, Trauner et al (2008) Wavelength (nm) Light Response Index
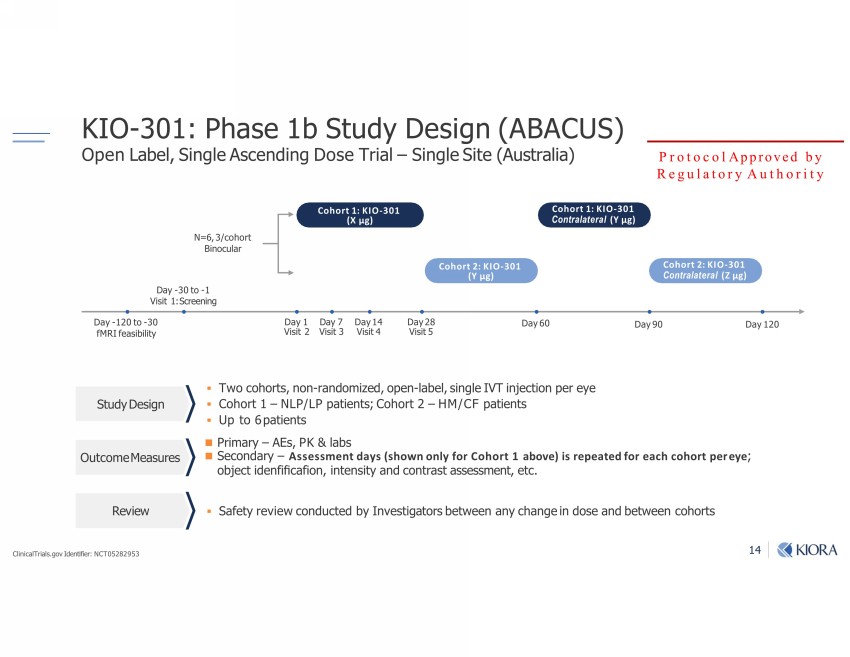
ƐƓ KIO - 301: Phase 1b Study Design (ABACUS) Open Label, Single Ascending Dose Trial – Single Site (Australia) ▪ Two cohorts, non - randomized, open - label, single IVT injection per eye ▪ Cohort 1 – NLP/LP patients; Cohort 2 – HM/CF patients ▪ Up to 6 patients Safety review conducted by Investigators between any change in dose and between cohorts Study Design Outcome Measures Review -u Day 90 Cohort 1: KIO - 301 (X µg) -"1u;;mbm] Day 28 Visit 5 Day 6 0 Day 120 Cohort 1: KIO - 301 Contralateral (Y µg) Cohort 2: KIO - 301 Contralateral (Z µg) Day 1 Visit 2 (bvb| Day 14 Visit 4 Day - 120 to - 30 fMRI feasibility Protocol Approved by Regulatory Authority ClinicalTrials.gov Identifier: NCT05282953

ƐƔ KIO - 301 Next Steps Initiate Phase 1b Clinical Trial
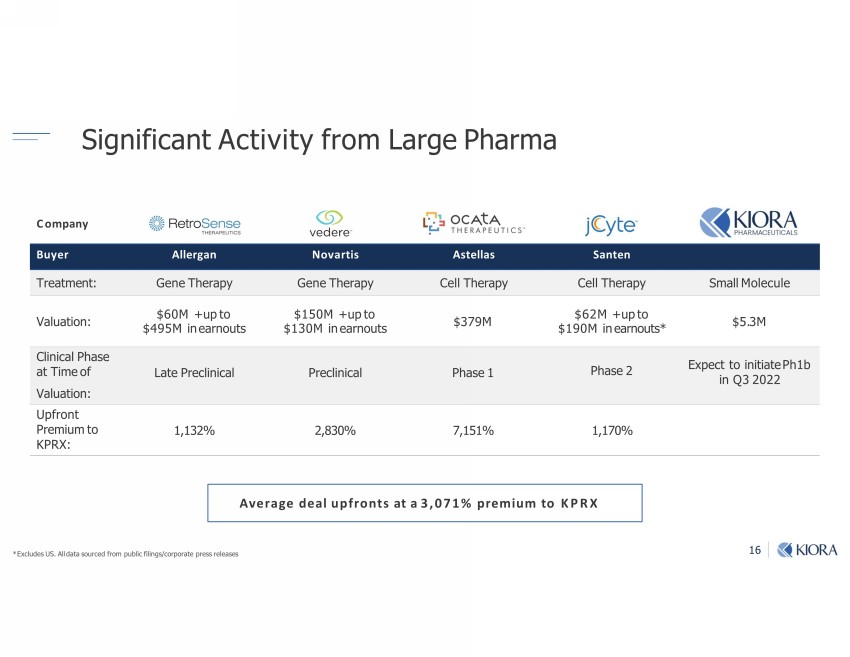
16 Significant Activity from Large Pharma ;u Allergan Novartis Astellas Santen Treatment: Gene Therapy Gene Therapy Cell Therapy Cell Therapy Small Molecule Valuation: $60M + up to $495M in earnouts $150M + up to $130M in earnouts $379M $62M + up to $190M in earnouts* $5.3M Clinical Phase at Time of Valuation: Late Preclinical Preclinical Phase 1 Phase 2 Expect to initiate Ph1b in Q3 2022 Upfront Premium to KPRX: 1,132% 2,830% 7,151% 1,170% Average deal upfronts at a 3,071% premium to KPRX Company * Excludes US. All data sourced from public filings/corporate press releases

17 KIO - 101 Target Population: Ocular Presentation of Rheumatoid Arthritis (OPRA) Product: Ocular DHODH Inhibitor IP: Anticipated through 2039
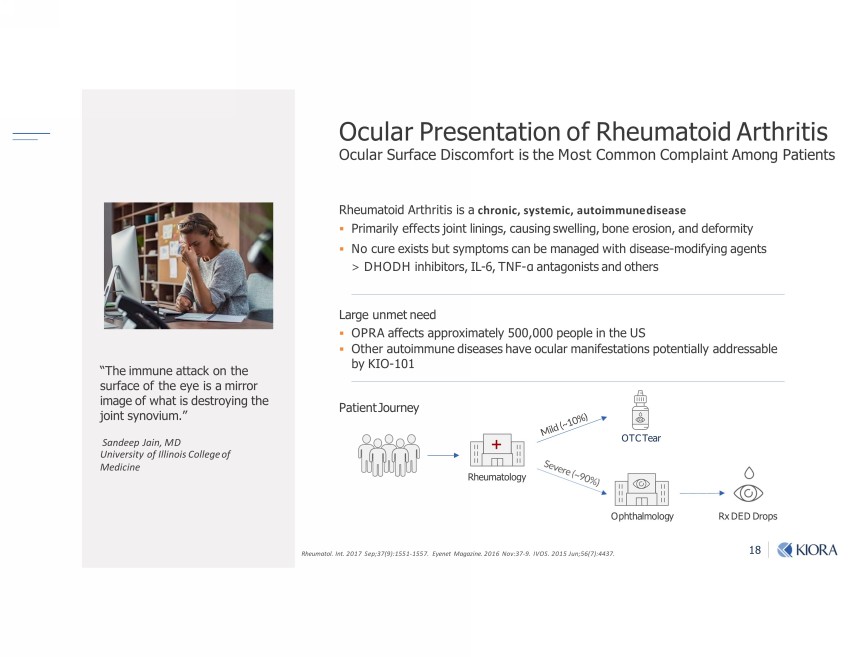
18 Ocular Presentation of Rheumatoid Arthritis Ocular Surface Discomfort is the Most Common Complaint Among Patients Large unmet need ▪ OPRA affects approximately 500,000 people in the US ▪ Other autoimmune diseases have ocular manifestations potentially addressable by KIO - 101 Patient Journey Rheumatoid Arthritis is a chronic, systemic, autoimmune disease ▪ Primarily effects joint linings, causing swelling, bone erosion, and deformity ▪ No cure exists but symptoms can be managed with disease - modifying agents ˃ DHODH inhibitors, IL - 6, TNF - α antagonists and others Rheumatol. Int. 2017 Sep;37(9):1551 - 1557. Eyenet Magazine. 2016 Nov:37 - 9. IVOS. 2015 Jun;56(7):4437. “The immune attack on the surface of the eye is a mirror image of what is destroying the joint synovium.” Sandeep Jain, MD University of Illinois College of Medicine OTC Tear Rx DED Drops Rheumatology Ophthalmology
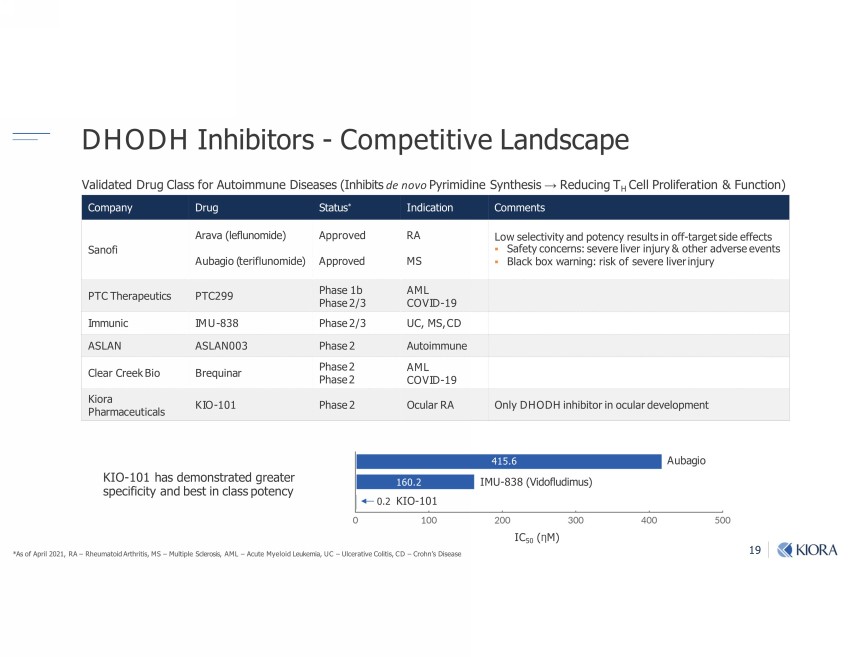
19 *As of April 2021, RA – Rheumatoid Arthritis, MS – Multiple Sclerosis, AML – Acute Myeloid Leukemia, UC – Ulcerative Colitis, CD – Crohn’s Disease KIO - 101 has demonstrated greater specificity and best in class potency Company Drug Status * Indication Comments Sanofi Arava (leflunomide) Approved RA Low selectivity and potency results in off - target side effects ▪ Safety concerns: severe liver injury & other adverse events ▪ Black box warning: risk of severe liver injury Aubagio (teriflunomide) Approved MS PTC Therapeutics PTC299 Phase 1b Phase 2/3 AML COVID - 19 Immunic IMU - 838 Phase 2/3 UC, MS, CD ASLAN ASLAN003 Phase 2 Autoimmune Clear Creek Bio Brequinar Phase 2 Phase 2 AML COVID - 19 Kiora Pharmaceuticals KIO - 101 Phase 2 Ocular RA Only DHODH inhibitor in ocular development Validated Drug Class for Autoimmune Diseases (Inhibits de novo Pyrimidine Synthesis → Reducing T H Cell Proliferation & Function) IMU - 838 (Vidofludimus) Aubagio KIO - 101 415.6 160.2 0.2 IC 50 ( Ƞ M) DHODH Inhibitors - Competitive Landscape
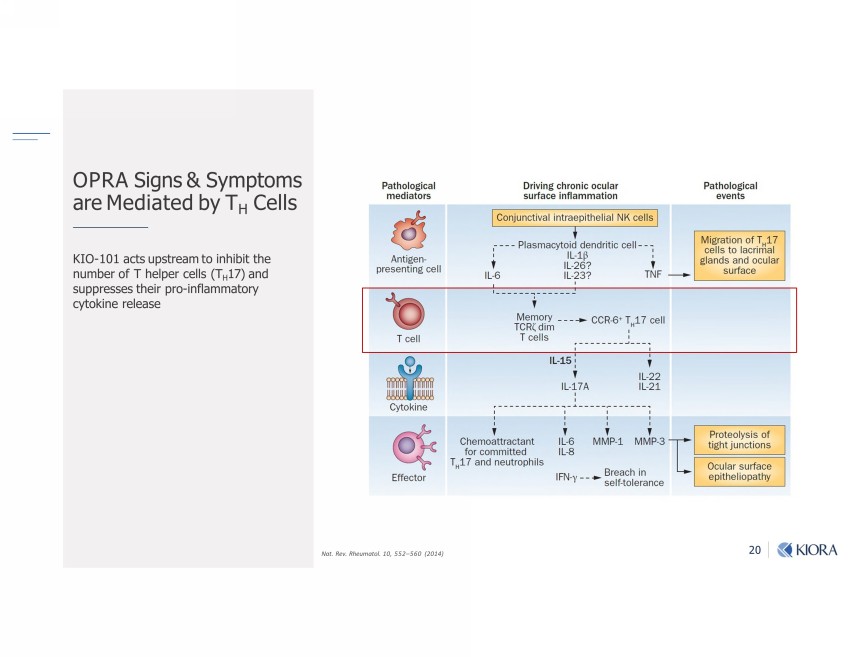
20 KIO - 101 acts upstream to inhibit the number of T helper cells (T H 17) and suppresses their pro - inflammatory cytokine release Nat. Rev. Rheumatol. 10, 552 – 560 (2014) OPRA Signs & Symptoms are Mediated by T H Cells
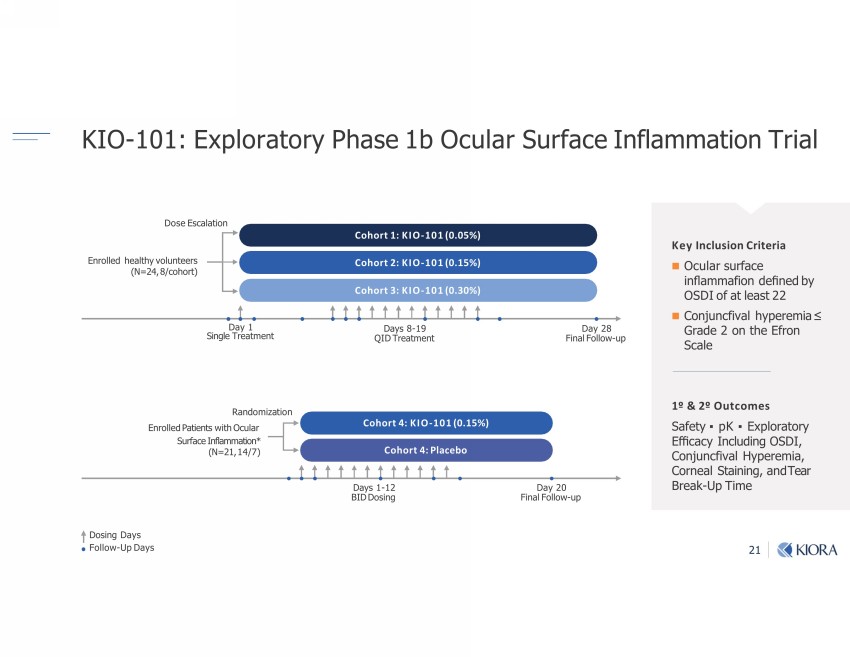
21 KIO - 101: Exploratory Phase 1b Ocular Surface Inflammation Trial Enrolled Patients with Ocular Surface Inflammation* (N=21, 14/7) Randomization Days 1 - 12 BID Dosing Day 20 Final Follow - up Cohort 4: KIO - 101 (0.15%) Cohort 4: Placebo Enrolled healthy volunteer s (N=24, 8/cohort) Dose Escalation Day 28 Final Follow - up Cohort 1: KIO - 101 (0.05%) Cohort 2: KIO - 101 (0.15%) Day 1 Single Treatment Days 8 - 19 QID Treatment Cohort 3: KIO - 101 (0.30%) Dosing Days Follow - Up Days
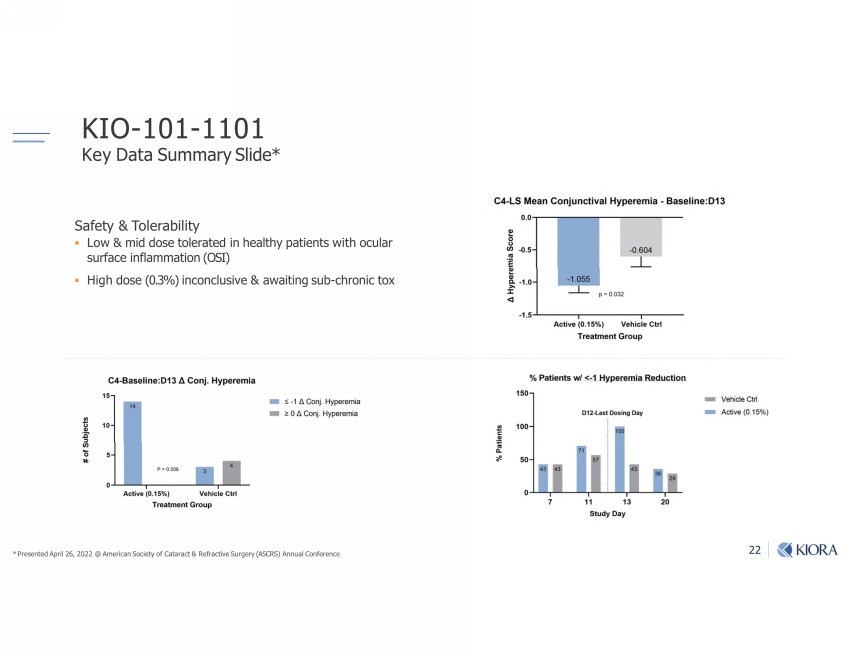
22 KIO - 101 - 1101 Key Data Summary Slide* Safety & Tolerability ▪ Low & mid dose tolerated in healthy patients with ocular surface inflammation (OSI) ▪ High dose (0.3%) inconclusive & awaiting sub - chronic tox * Presented April 26, 2022 @ American Society of Cataract & Refractive Surgery (ASCRS) Annual Conference
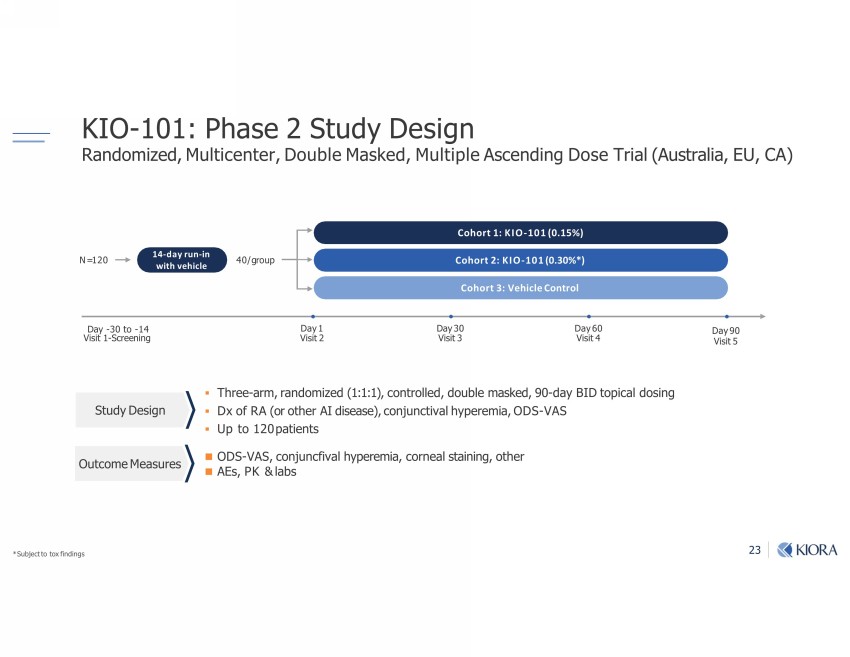
23 KIO - 101: Phase 2 Study Design Randomized, Multicenter, Double Masked, Multiple Ascending Dose Trial (Australia, EU, CA) ▪ Three - arm, randomized (1:1:1), controlled, double masked, 90 - day BID topical dosing ▪ Dx of RA (or other AI disease), conjunctival hyperemia, ODS - VAS ▪ Up to 120 patients " Ŋ ("ķ1omfm1ঞ-Ѵ_r;u;lb-ķ1oum;-Ѵv|-bmbm]ķo|_;u v ķ ş Ѵ-0v "|7 ;vb]m N= 120 Day 90 Visit 5 Cohort 1: KIO - 101 (0.15%) Cohort 2: KIO - 101 (0.30%*) Day 1 Visit 2 Cohort 3: Vehicle Control - Ŋ ƒƏ |o Ŋ ƐƓ (bvb| Ɛ Ŋ "1u;;mbm] - ƒƏ (bvb| ƒ Day 6 0 Visit 4 14 - day run - in with vehicle 40/ group * Subject to tox findings Outcome Measures
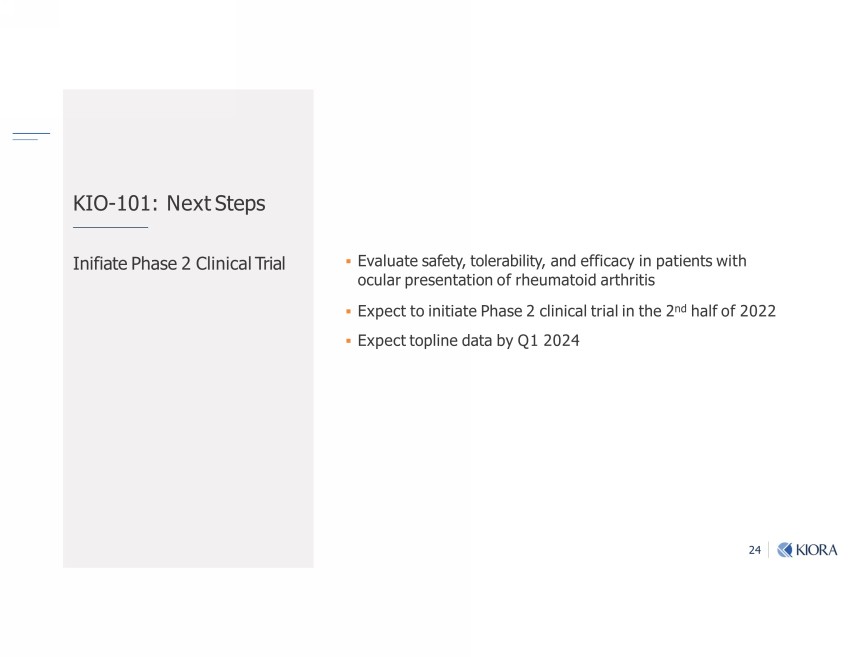
24 Ŋ ƐƏƐ Ĺ;|"|;rv mbঞ-|;_-v; Ƒ Ѵbmb1-Ѵ$ub-Ѵ ▪ Evaluate safety, tolerability, and efficacy in patients with ocular presentation of rheumatoid arthritis ▪ Expect to initiate Phase 2 clinical trial in the 2 nd half of 2022 ▪ Expect topline data by Q1 2024

25 KIO - 201 Target Population: Ocular Surface Wound Healing Product: Synthetic Modified Hyaluronic Acid IP: Through 2034* * Subject to 7 years regulatory exclusivity if ODD granted
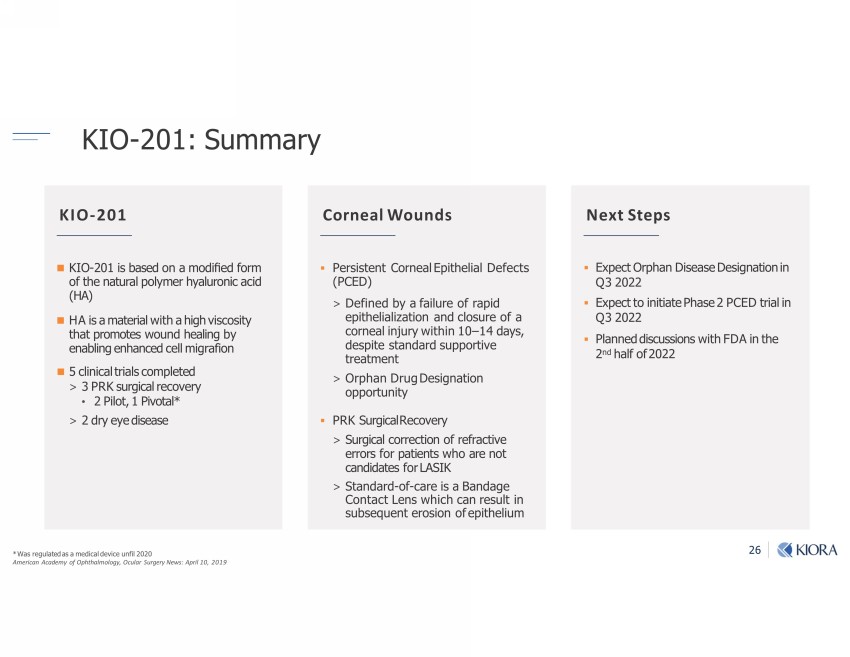
26 KIO - 201: Summary Ŗ)-vu;]Ѵ-|;7-v-l;7b1-Ѵ7;b1;mঞѴ ƑƏƑƏ l;ub1-m1-7;lo=r_|_-ѲloѲo]Ķ1Ѳ-u"u];u;vثrubѲ Ǝƍ Ķ ƏƍƎƔ Ŋ ƑƏƐ bv0-v;7om-lo7bC;7=oul o=|_;m-|u-ѴroѴl;u_-Ѵuomb1-1b7 Őő bv-l-|;ub-Ѵb|_-_b]_bv1ovb| |_-|ruolo|;vom7_;-Ѵbm]0 ;m-0Ѵbm];m_-m1;71;ѴѴlb]u-ঞom Ɣ 1Ѵbmb1-Ѵ|ub-Ѵv1olrѴ;|;7 ૩ ƒ !vu]b1-Ѵu;1o;u Ƒ bѴo|ķ Ɛ bo|-ѴŖ ૩ Ƒ 7 u;;7bv;-v; ▪ Persistent Corneal Epithelial Defects (PCED) ˃ Defined by a failure of rapid epithelialization and closure of a corneal injury within 10 – 14 days, despite standard supportive treatment ˃ Orphan Drug Designation opportunity ▪ PRK Surgical Recovery ˃ Surgical correction of refractive errors for patients who are not candidates for LASIK ˃ Standard - of - care is a Bandage Contact Lens which can result in subsequent erosion of epithelium ▪ Expect Orphan Disease Designation in Q3 2022 ▪ Expect to initiate Phase 2 PCED trial in Q3 2022 ▪ Planned discussions with FDA in the 2 nd half of 2022 KIO - 201 Corneal Wounds Next Steps
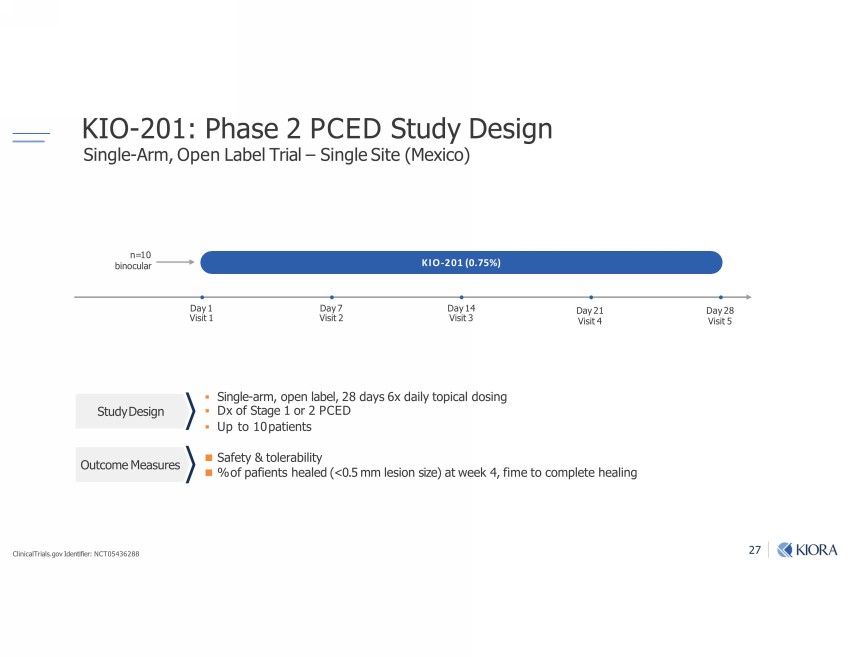
27 Ŋ ƑƏƐ Ĺ_-v; Ƒ "|7 ;vb]m "bm]Ѵ; Ŋ ulķr;m-0;Ѵ$ub-Ѵ ŋ "bm]Ѵ;"b|;Ő;b1oő - ƑƐ (bvb| Ɠ KIO - 201 (0.75%) Day 1 Visit 1 Day 7 Visit 2 Day 14 Visit 3 ▪ Single - arm, open label, 28 days 6x daily topical dosing ▪ Dx of Stage 1 or 2 PCED ▪ Up to 10 patients "-=;| ş |oѴ;u-0bѴb| ѷo=r-ঞ;m|v_;-Ѵ;7Őƺ ƏĺƔ llѴ;vbomvb;ő-|;;h Ɠ ķঞl;|o1olrѴ;|;_;-Ѵbm] "|7 ;vb]m |1ol;;-vu;v n=10 binocular Day 28 Visit 5 ClinicalTrials.gov Identifier: NCT05436288

28 KIO - 201: Next Steps mbঞ-|;_-v; Ƒ Ѵbmb1-Ѵ$ub-Ѵ ;uvbv|;m|oum;-Ѵrb|_;Ѵb-Ѵ ;=;1|v mbঞ-|;7_-v; Ƒ 1Ѵbmb1-Ѵ|ub-Ѵbm ƑƑƏƑƑ r;1||orѴbm;7-|-bm ƐƑƏƑƒ ur_-m u] ;vb]m-ঞom;r;1|;7bm ƒƑƏƑƑ bv1vvbomvb|_ ;r;1|;7bm|_; Ƒ m7 _-Ѵ=o= ƑƏƑƑ
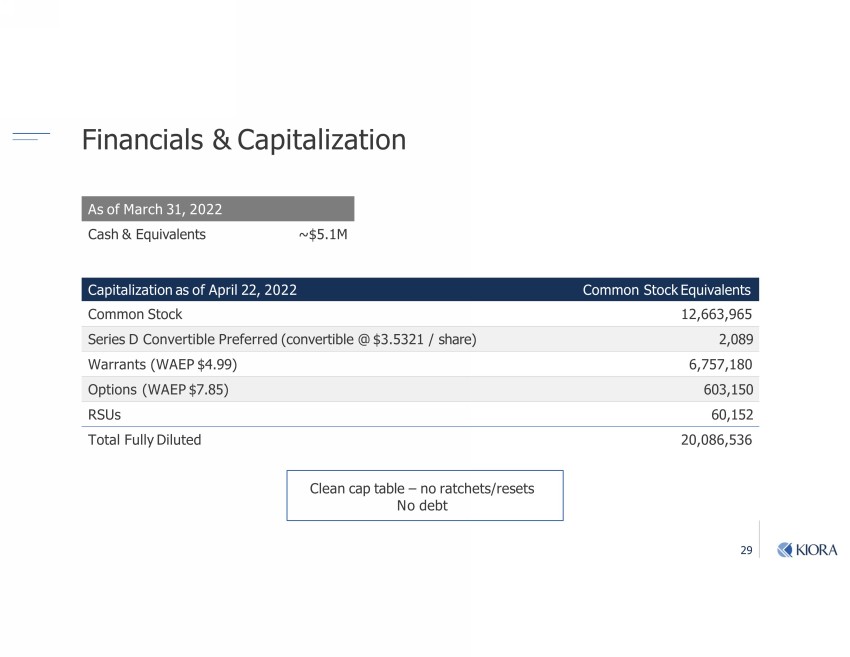
29 Financials & Capitalization Capitalization as of April 22, 2022 Common Stock Equivalents Common Stock 12,663,965 Series D Convertible Preferred (convertible @ $3.5321 / share) 2,089 Warrants (WAEP $4.99) 6,757,180 Options (WAEP $7.85) 603,150 RSUs 60,152 Total Fully Diluted 20,086,536 As of Dec 31, 2021 -v_ştb-Ѵ;m|v ~$7.9M Clean cap table – no ratchets/resets No debt

30 Leadership Team Brian M Strem, PhD President & CEO Susan Drexler, CPA Financial Consultant "|;=-m"r;uѴķ_ ( ŋ şr;u-ঞomv ub1 -mb;Ѵvķ ķ _b;= ;;Ѵorl;m|L1;u Angela Dentiste, MBA VP – Clinical Operations

31 Board of Directors Brian M Strem, PhD President & CEO Paul Chaney Chairman Aron Shapiro ;m-uom u-;;m$Ѵ; David Hollander, MD, MBA Erin Parsons

32 Scientific Advisory Board Daniel Durrie, MD Russel Van Gelder, MD, PhD _-uѴb;)ho@ķ (b1|ou;u;ķ Francis Mah, MD Paul Karpecki, OD, FAAO Allen Ho, MD, PhD Christine Kay, MD, PhD Vitreo Retinal ASSOCIATES
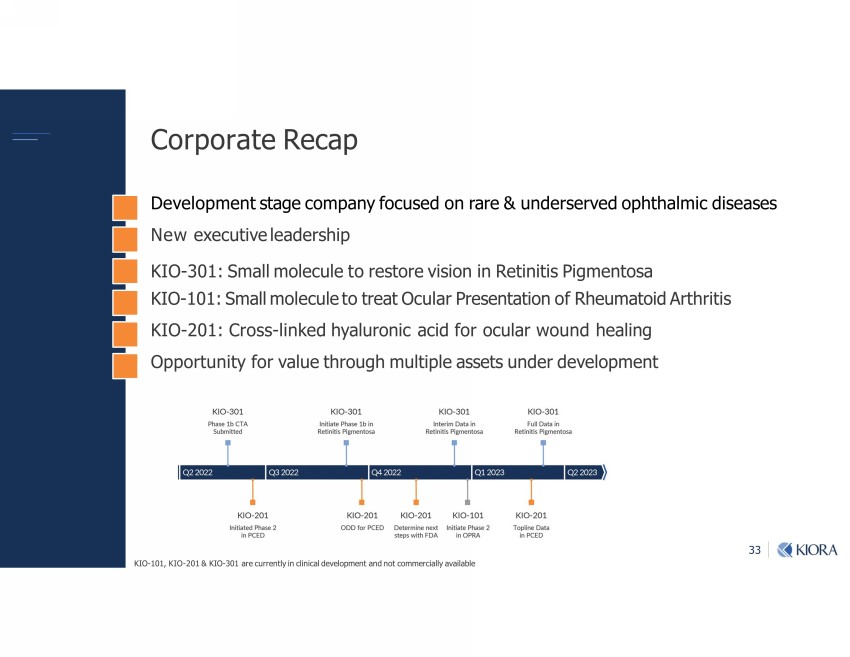
33 ourou-|;!;1-r ;;Ѵorl;m|v|-];1olr-m=o1v;7omu-u;şm7;uv;u;7or_|_-Ѵlb17bv;-v;v New executive leadership KIO - 301: Small molecule to restore vision in Retinitis Pigmentosa KIO - 101: Small molecule to treat Ocular Presentation of Rheumatoid Arthritis KIO - 201: Cross - linked hyaluronic acid for ocular wound healing Opportunity for value through multiple assets under development KIO - 101, KIO - 201 & KIO - 301 are currently in clinical development and not commercially available

om|-1|Ĺ bm=oŠhbou-r_-ul-ĺ1ol